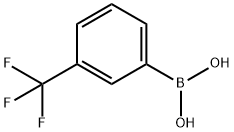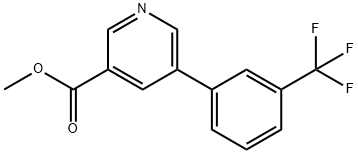
methyl 5-(3-(trifluoromethyl)phenyl)pyridine-3-carboxylate synthesis
- Product Name:methyl 5-(3-(trifluoromethyl)phenyl)pyridine-3-carboxylate
- CAS Number:893734-85-5
- Molecular formula:C14H10F3NO2
- Molecular Weight:281.23
Yield:-
Reaction Conditions:
with potassium phosphate;tetrakis(triphenylphosphine) palladium(0) in 1,4-dioxane;water at 100; for 6 h;
Steps:
11.1
Example 11; Preparation of c?-l-(4-inethyl-6-(ljff-pyrazol-5-ylamino)pyrimidin~2-yl]-5-[3-(trifluoroniethyl)phenyl]piperdine-3-carboxylic acid trifluoroacetate Step 1: Preparation of methyl 5-[3-(trifluoromethyl)phenyl]nicotmateMethyl 5-bromonicotinate (168.2 mg), 3-trifluoromethylbenzeneboromc acid (165.6 mg), K3PO4 (378.5 mg) and Pd(PPh3)4 (92.2 mg) was mixed in dioxane (5 ml). To the suspension was added water (0.25 ml). And the mixture was heated to 100 0C under stirring. After 6 hours, the mixture was cooled to room temperature and was diluted with EtOAc. The insoluble material was filtered off and washed with EtOAc. The filtrate was poured into saturated aqueous solution OfNaHCO3 and extracted with EtOAc. The extract was washed with brine, dried over MgSO4 and concentrated. The residue was purified with silica gel column chromatography (eluent : hexane / EtOAc - 94 / 6 ~ 50 / 50) to give methyl 5-[3- (trifluoromethyl)phenyl]nicotmate (190.1 mg) as a pale yellow solid.1H NMR (400 MHz3 CDCl3) δ 4.01 (3H, s), 7.65 (IH, t), 7.71 (IH, d), 7.81 (IH, d), 7.87 (IH, s), 8.51 (IH, t), 9.02 (IH, d), 9.25 (IH, d) ESI-MS m/z 282.1 [M+Hf.
References:
WO2010/111056,2010,A1 Location in patent:Page/Page column 38-39