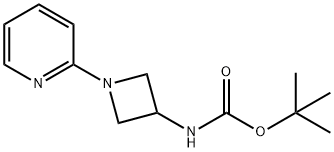
tert-Butyl N-[1-(pyridin-2-yl)azetidin-3-yl]carbamate synthesis
- Product Name:tert-Butyl N-[1-(pyridin-2-yl)azetidin-3-yl]carbamate
- CAS Number:898271-45-9
- Molecular formula:C13H19N3O2
- Molecular Weight:249.31
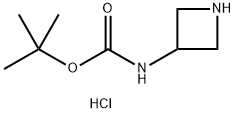
217806-26-3
164 suppliers
$12.00/1g
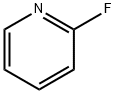
372-48-5
368 suppliers
$5.00/5g
![tert-Butyl N-[1-(pyridin-2-yl)azetidin-3-yl]carbamate](/CAS/20140602/GIF/CB42698287.gif)
898271-45-9
12 suppliers
$503.00/1g
Yield:898271-45-9 15%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 85;
Steps:
A A. tert-Butyl (1 -(pyridin-2-yI)azetidin-3-yI)carbamate
To terf-butyl azetidin-3-ylcarbamate hydrochloride (500 mg, 2.40 mmol) in DMF (3 mL) was added N,N-diisopropylethylamine (0.84 mL, 4.8 mmol) followed by 2-fluoropyridine (233 mg, 2.40 mmol). The mixture was heated at 85 °C for overnight, cooled, diluted with EtOAc, washed with water, and the aqueous extracted with EtOAc. The combined organic layers were washed with water (2X) and brine, dried over MgSC , filtered and concentrated. The residue was purified on silica gel eluting with a 20%-70% EtOAc in hexanes gradient. The appropriate fractions were combined, evaporated under reduced pressure and placed in vacuo to give the title compound as a white solid (90 mg, 15%). 1 H NMR (400 MHz, CDCI3) δ 1 .49 (s, 9 H), 3.82 (dd, J = 8, 5 Hz, 2 H), 4.38 (t, J = 8 Hz, 2 H), 4.66 (s br, 1 H), 6.33 (d, J = 8 Hz, 1 H), 6.61 -6.70 (m, 1 H), 7.42-7.53 (m, 1 H), 8.18 (d, J = 4 Hz, 1 H); LC-MS (LC-ES) M+H = 250.
References:
WO2018/69863,2018,A1 Location in patent:Page/Page column 147