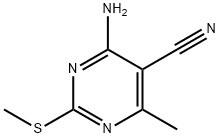
4-Amino-6-methyl-2-(methylsulfanyl)-pyrimidine-5-carbonitrile synthesis
- Product Name:4-Amino-6-methyl-2-(methylsulfanyl)-pyrimidine-5-carbonitrile
- CAS Number:89853-27-0
- Molecular formula:C7H8N4S
- Molecular Weight:180.23
Yield:89853-27-0 74%
Reaction Conditions:
with sodium methylate in methanol at 0 - 20;
Steps:
4-amino-6-methyl-2-(methylthio)-5-pyrimidinecarbonitrile (5)
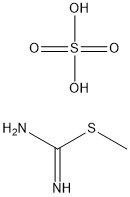
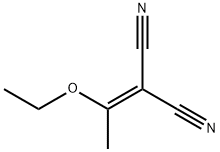
To a mixture of 2-(1-ethoxyethylidene)malononitrile (19.6 g, 144 mmol) and S-methlylisothiourea hemisulfate salt (30.2 g, 230 mmol) was added sodium methoxide (7.78 g, 144 mmol) at 0 °C. The mixture was warmed up to room temperature and stirred overnight. The reaction was cooled to 0 °C and quenched with water (900 mL) and was stirred for additional 30 min. The resulting precipitate was collected by filtration, washed with water, and dried under vacuum to give 4-amino-6-methyl-2-(methylthio)pyrimidine-5-carbonitrile (19.2 g,74 % yield) the desired product as a white solid. MS (ES) m/z = 181.2 [M+H]+; 1H NMR (400 MHz, DMSO-d6) δ 2.36 (s, 3H), 2.44 (s, 3H), 7.75 (br.s. 2H).
References:
Tian, Xinrong;Suarez, Dominic P.;Li, William Hoi Hong;McSherry, Allison K.;Sanchez, Robert M.;Moore, Michael L.;Axten, Jeffrey M. [Tetrahedron Letters,2019,vol. 60,# 49,art. no. 151312] Location in patent:supporting information