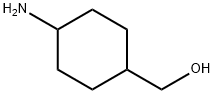
4-(Hydroxymethyl)cyclohexan-1-amine, 1-Amino-4-(hydroxymethyl)cyclohexane synthesis
- Product Name:4-(Hydroxymethyl)cyclohexan-1-amine, 1-Amino-4-(hydroxymethyl)cyclohexane
- CAS Number:89854-94-4
- Molecular formula:C7H15NO
- Molecular Weight:129.2
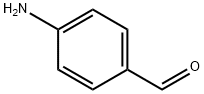
62-23-7
674 suppliers
$14.00/25g

89854-94-4
20 suppliers
inquiry
Yield:89854-94-4 90.1 %Chromat.
Reaction Conditions:
with hydrogen in water at 220; under 37503.8 Torr; for 24 h;Autoclave;
Steps:
2.3. Catalytic tests
General procedure: Before the reaction test, the catalyst was activated in 10 vol%H2/Ar at 20 mL/min in 300 C for 2 h. Unless otherwise specified,1 mmol substrate, 0.05 g catalyst and 5 mL H2O were charged intoa stainless steel autoclave (NS10316L, Anhui Kemi MachineryTechnology Co., Ltd.). The reactor was sequentially purged withH2 for 5 times, pressured to 5.0 MPa H2, heated into 220 C, andkept at this temperature for 24 h. After the reaction, the reactionsystem was quickly cooled to room temperature in an ice-waterbath. After separation, the liquid products were analyzed by gaschromatography (Shimadzu GC-2010) with a flame ionizationdetector. 1,2-butanediol was used as an internal standard for theformation of diol, while 1-butanol was applied in the case of monohydricalcohol. The insoluble reactants and products in water were extracted by ethyl acetate, and analyzed with ethyl benzoate asinternal standard. Diethyl ether was used as extract in the conversion of stearic acid and palmitic acid. The gas products were analyzedby Agilent 7890A gas chromatograph equipped with a TCDdetector. All the products were identified by a Shimadzu GCMSQP2010gas chromatogram-mass spectrometer (GC-MS) andNMR spectroscopy (Bruker AV-III 400 MHz NMR spectrometer).
References:
Dong, Mei;Fan, Weibin;Gao, Xiaoqing;Zhu, Shanhui [Journal of Catalysis,2021,vol. 399,p. 201 - 211]

555-16-8
532 suppliers
$5.00/5g

89854-94-4
20 suppliers
inquiry

62456-15-9
66 suppliers
$29.00/250mg

89854-94-4
20 suppliers
inquiry
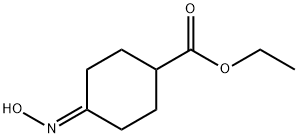
90942-89-5
14 suppliers
inquiry

89854-94-4
20 suppliers
inquiry