
9,10-bis(6-phenylpyridin-3-yl)anthracene synthesis
- Product Name:9,10-bis(6-phenylpyridin-3-yl)anthracene
- CAS Number:1257879-34-7
- Molecular formula:C36H24N2
- Molecular Weight:484.59
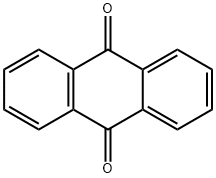
84-65-1
543 suppliers
$5.00/100mg
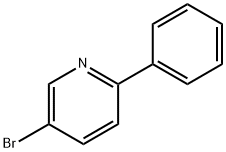
27012-25-5
169 suppliers
$38.00/250mg

1257879-34-7
20 suppliers
inquiry
Yield:1257879-34-7 20.48%
Reaction Conditions:
Stage #1: 5-bromo-2-phenylpyridinewith n-butyllithium in tetrahydrofuran at -70; for 0.166667 h;Inert atmosphere;
Stage #2: 9,10-phenanthrenequinone in tetrahydrofuran;
Stage #3: with sodium phosphinate;water;acetic acid;potassium iodide for 1 h;Reflux;
Steps:
3.2
(2) Reaction of the second step [Show Image] After nitrogen replacement in a three-neck bottle of 500 ml equipped with magnetic stirrer, 6.75 g of 2-phenyl-5-bromopyridine (purity of 95.45%, 0.0274 mol) and 110 ml of THF are sequentially added thereto. 13 ml of n-butyl lithium (concentration of 2.5 M, 0.0325 mol) is dropwise added at -70°C. After agitation for 10 minutes, 2.6 g of anthraquinone (purity of 99%, 0.0124 mol) is added. After addition, the temperature is naturally raised to room temperature, and the solution exhibits bright yellow color. 200 ml of water is added for hydrolysis, the resultant is extracted with ethyl acetate, and the solvent is evaporated. After adding 220 ml of acetic acid, 22 g of KI and 22 g of sodium hypophosphite, the resultant is refluxed and allowed to react for 1 hour. Then, the temperature is reduced, and the resultant is filtered to obtain 2.8 g of pale yellow product. The pale yellow product is boiled in water/THF with a ratio of 15/100, cooled and filtered, which are repeated for several times. Thus, 2.1g of pale white compound 2-5 is obtained with purity of 99.0% and yield of 20.48%. Mass Spectrum of the product (MS) (m/e): 484; Elemental analysis (C36H24N2): Theoretical, C: 89.23%, H: 4.99%, N: 5.78%; Measured, C: 89.30%, H: 5.01%, N: 5.69%.
References:
EP2500343,2012,A1 Location in patent:Page/Page column 12