9,11-Dihydroxy-12H-benzo[a]xanthen-12-one synthesis
- Product Name:9,11-Dihydroxy-12H-benzo[a]xanthen-12-one
- CAS Number:53865-04-6
- Molecular formula:C17H10O4
- Molecular Weight:278.26
Yield:53865-04-6 7.23%
Reaction Conditions:
with zinc(II) chloride;trichlorophosphate at 80; for 5 h;
Steps:
14.1; 15.1 Step 1:
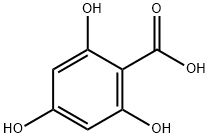
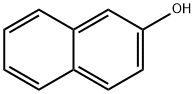
Step 1: Synthesis of 9,11-dihydroxy-12H-benzo[a]xanthen-12-one A mixture of 2,4,6-trihydroxybenzoic acid (1.88 g, 0.01 mol), 2-naphthol (1.44 g, 0.01 mol), ZnCl2 (5.0 g, 0.036 mol) and POCl3 (40 mL) was stirred under reflux at 80° C. for 5 hours. The reaction liquid was cooled to room temperature, and was very slowly added to 1 L of ice water. When the reaction liquid was poured in ice water, ice was added portionwise to prevent overheating. The resulting solid was allowed to stand (1 d), filtered, washed with water, and then dried under reduced pressure to obtain a red compound. This compound was separated and purified by silica gel column chromatography (developing solvent: ethyl acetate:n-hexane=1:3 (v/v)) to give the title compound (202 mg, 7.23%) as a yellow solid. Rf: 0.28 (ethyl acetate:n-hexane=1:3 (v/v)); 1H-NMR (400 MHz, CDCl3) δ 6.26 (s, 1H), 6.38 (s, 1H), 7.42 (d, J=8.8 Hz, 1H), 7.52 (dd, J=7.6, 6.8 Hz, 1H), 7.69 (dd, J=7.2, 7.2 Hz, 1H), 7.83 (d, J=8.0 Hz, 1H), 8.04 (d, J=8.8 Hz, 1H), 9.87 (d, J=8.4 Hz, 1H).
References:
US2012/190724,2012,A1 Location in patent:Page/Page column 11-12