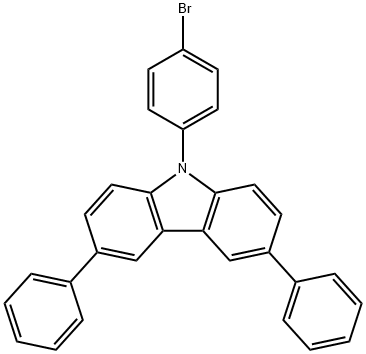
9-(4-bromophenyl)-3,6-diphenyl-9H-carbazole synthesis
- Product Name:9-(4-bromophenyl)-3,6-diphenyl-9H-carbazole
- CAS Number:607739-92-4
- Molecular formula:C30H20BrN
- Molecular Weight:474.39
Yield:607739-92-4 85%
Reaction Conditions:
with copper (I) iodide;(1S,2S)-(+)-1,2-diaminocyclohexane;sodium tertiary butoxide in 1,4-dioxane at 100; for 48 h;
Steps:
3 Synthesis of 3-Br:
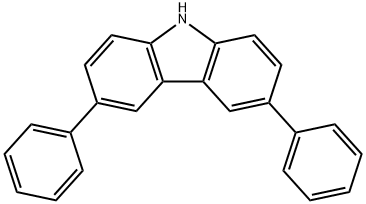
3,6-diphenylcarbazole (3.33g, 10.42mmol, 1.0 equivalent), 1-bromo-4-iodobenzene (3.24g, 11.46mmol, 1.1 equivalent), cuprous iodide (39.7 mg, 0.21 mmol, 0.02 equivalent) and sodium tert-butoxide (2.00 g, 20.81 mmol, 2.0 equivalent) were sequentially added to a dry three-necked flask equipped with magnetic stirring.Purge nitrogen for three times, then add 1,2-trans-cyclohexanediamine (0.13mL, 1.04mmol, 0.1 equivalent) and 1,4-dioxane (100mL) under nitrogen protection, and place the mixture in an oil bath And stir the reaction at 100°C.It was monitored by thin layer chromatography until the reaction was completed, and the reaction was stopped after 48 hours.The resulting mixture was cooled to room temperature and quenched by adding water.The mixture was extracted three times with dichloromethane.The combined organic layer was washed with water, dried over anhydrous sodium sulfate and filtered.The filtrate was concentrated under reduced pressure, and the sample was purified by silica gel column chromatography (eluent: petroleum ether) to obtain 4.20 g of a white solid with a yield of 85%.
References:
CN111471043,2020,A Location in patent:Paragraph 0066-0068