
9,9-diphenyl-9,10-dihydroacridine synthesis
- Product Name:9,9-diphenyl-9,10-dihydroacridine
- CAS Number:20474-15-1
- Molecular formula:C25H19N
- Molecular Weight:333.43
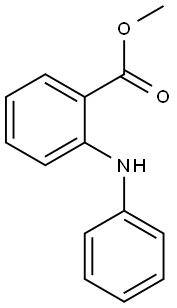
35708-19-1
40 suppliers
$334.62/5g
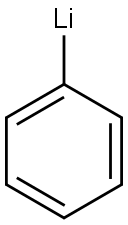
591-51-5
128 suppliers
$45.89/50ml

20474-15-1
74 suppliers
$61.00/100mg
Yield: 73%
Reaction Conditions:
Stage #1:Methyl N-phenylanthranilate;phenyllithium in tetrahydrofuran at -78; for 1 h;Inert atmosphere;
Stage #2: with methyl bisulfate in chloroform for 1 h;Reflux;
Steps:
7.1 (1) Synthesis of Intermediate 25(1)
1.0 g (1.0 eq, 4.4 mmol) of methyl 2-(phenylamino)benzoate was added to the reaction vessel and vacuum dried, and the reaction vessel was filled with nitrogen. 17 mL of THF was added to the reaction vessel and stirred to dissolve the compound, and the reaction vessel was cooled to a temperature of -78°C. 1.9M phenyllithium (3.0eq, 7.0mL) was slowly added dropwise thereto, and stirred at -78°C for 1 hour. The reaction solution was stirred at a temperature of 0°C for 2 hours, and then additionally stirred at room temperature for 4 hours. After the completion of the reaction, the reaction product was washed with distilled water, and the organic layer was extracted therefrom by using chloroform. The extracted organic layer was dried by using anhydrous magnesium sulfate, and filtered through Celite, and the solvent was evaporated therefrom. The dried reaction product was added to the reaction vessel, and 20 mL of chloroform was added thereto to dissolve the compound. 1 mL of methanesulfonic acid (MSA) was added thereto, and stirred under reflux for 1 hour. After the reaction was completed, the reaction product was neutralized by using an aqueous sodium hydrogen carbonate solution, and the organic layer was extracted therefrom by using distilled water and chloroform. The extracted organic layer was dried by using anhydrous magnesium sulfate, and filtered through Celite, and the solvent was evaporated therefrom. After column chromatography, it was recrystallized using chloroform and hexane to obtain 1.1 g (yield 73percent) of intermediate 25(1) (ie 9,9-diphenyl-9,10-dihydroacridine).
References:
Sanxing Display Co., Ltd.;Chengjunguan University Xiaochan Xue Xieli Tuan;Po Huizhen;Yin Shengzhu;Zheng Huiren CN111333615, 2020, A Location in patent:Paragraph 0619-0623

230627-85-7
0 suppliers
inquiry

20474-15-1
74 suppliers
$61.00/100mg

119-61-9
793 suppliers
$5.00/10g
61613-22-7
93 suppliers
$11.00/250mg

20474-15-1
74 suppliers
$61.00/100mg

35708-19-1
40 suppliers
$334.62/5g
100-58-3
190 suppliers
$18.00/10ml

20474-15-1
74 suppliers
$61.00/100mg

35708-19-1
40 suppliers
$334.62/5g

20474-15-1
74 suppliers
$61.00/100mg