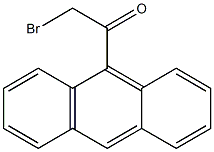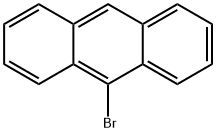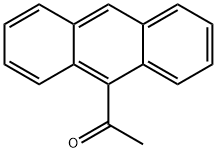
9-ACETYLANTHRACENE synthesis
- Product Name:9-ACETYLANTHRACENE
- CAS Number:784-04-3
- Molecular formula:C16H12O
- Molecular Weight:220.27
Yield:784-04-3 94% ,1564-64-3 2%
Reaction Conditions:
with diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate;acetic acid for 0.7 h;Irradiation;Inert atmosphere;
Steps:
Reduction of α-Haloketones 2; General Procedure
General procedure: Into a flask charged with the respective α-haloketone 2 (1 mmol) and ethidine (Hantzsch's ester, 1a; 1.2 mmol), was added the solvent as per Tables 2-4 (2.5 mL). The flask was then attached to a balloon filled with N2 and irradiated with 3 W blue LED at a distance of 5 cm. The workup was followed when TLC showed that ethidine or the haloketone was consumed. When AcOH was used as the solvent, the reaction was worked up as follows: the reaction mixture was partitioned between EtOAc (40 mL) and H2O (10 mL), then the organic phase was washed with sat. aq NaHCO3 (3 15 mL) and brine (15 mL), and dried (anhyd Na2SO4). After concentration under reduced pressure, the residue was subjected to flash chromatography for purification eluting with petroleum ether (PE) and CH2Cl2. When aprotic polar solvents were used, the reaction mixture was diluted with EtOAc (40 mL) and the organic layer was washed with H2O (3 15 mL) and brine (15 mL), and dried (anhyd Na2SO4). When volatile solvents were used, the mixture was concentrated under reduced pressure and the residue was subjected to flash chromatography for purification.
References:
Lu, Zheng;Yang, Yong-Qing [Synthesis,2019,vol. 51,# 2,p. 508 - 515]

13752-40-4
26 suppliers
inquiry

784-04-3
122 suppliers
$35.10/5g
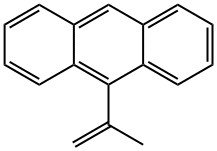
58873-48-6
1 suppliers
inquiry

784-04-3
122 suppliers
$35.10/5g
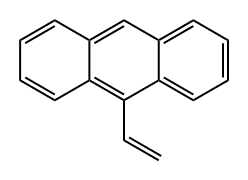
2444-68-0
167 suppliers
$12.00/250mg

784-04-3
122 suppliers
$35.10/5g