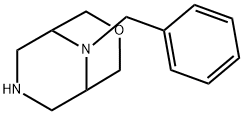
9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane synthesis
- Product Name:9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane
- CAS Number:864448-39-5
- Molecular formula:C13H18N2O
- Molecular Weight:218.29
![3-Benzyl-7-(Phenylsulfonyl)-9-Oxa-3,7-Diazabicyclo[3.3.1]Nonane](/CAS/20180703/GIF/335620-96-7.gif)
335620-96-7
7 suppliers
inquiry
![9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-39-5.gif)
864448-39-5
1 suppliers
inquiry
Yield:864448-39-5 84%
Reaction Conditions:
Stage #1: 9-benzyl-3-(phenylsulfonyl)-7-oxa-3,9-diaza-bicyclo[3.3.1]nonanewith sodium bis(2-methoxyethoxy)aluminium dihydride in toluene;xylene; for 2 h;Heating / reflux;
Stage #2: with hydrogenchloride;water in toluene;xylene;
Stage #3: with sodium hydroxide in water;
Steps:
102.b
b) 9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane; 7-Benzenesulfonyl-9-benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane (400mg; 1.1 mmol) is dissolved in xylene (8 ml), Red-AI (-3.5 M in toluene; 0.8 ml; 2.8 mmol) added and refluxed for 1.5 h. A second portion of Red-AI (0.4 ml; 1.4 mmol) is added and refluxed for another 30 min. The reaction mixture is poured on 2N HCI (100 ml) and washed twice with TBME. NaOH conc is added to the aqueous phase and extracted with TBME/EtOH (50: 1) three times. The combined organic phases are dried with K2C03, filtered, evaporated to dryness and purified via chromatography (Si02, EtOAc/MeOH/NH3conc 80/20/4) to yield the title compound as yellowish oil, which crystallised in needles (206 mg; 84 %). 1H-NMR (400MHz; DMSO-d6), No. (ppm) : 2.27 (s, 2.84 (d, 3.16 (bd, 3.79 (d, 3.98 (s, 4.03 (d, 7.13-7.40 (m, MS (m/z) ES+: 219.1 (MH+, 100).
References:
WO2005/103054,2005,A2 Location in patent:Page/Page column 134
![Propanoic acid, 3,3'-[(phenylsulfonyl)imino]bis[2-bromo-, diethyl ester, (2R,2'S)- (9CI)](/CAS/20211123/GIF/864448-25-9.gif)
864448-25-9
0 suppliers
inquiry
![9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-39-5.gif)
864448-39-5
1 suppliers
inquiry
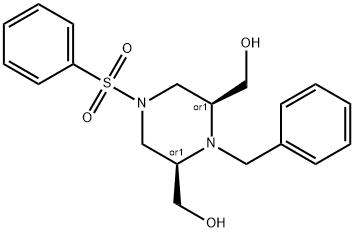
864448-27-1
0 suppliers
inquiry
![9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-39-5.gif)
864448-39-5
1 suppliers
inquiry
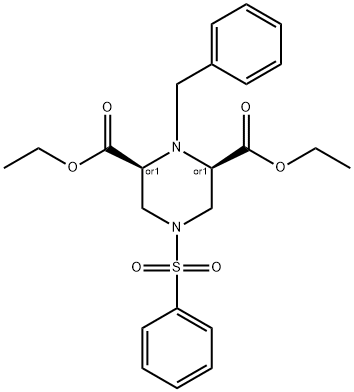
864448-26-0
0 suppliers
inquiry
![9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-39-5.gif)
864448-39-5
1 suppliers
inquiry

100-46-9
461 suppliers
$5.00/5G
![9-Benzyl-3-oxa-7,9-diazabicyclo[3.3.1]nonane](/CAS/20150408/GIF/864448-39-5.gif)
864448-39-5
1 suppliers
inquiry