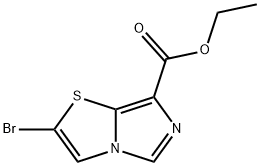
2-Bromo-Imidazo[5,1-B]Thiazole-7-Carboxylic Acid Ethyl Ester synthesis
- Product Name:2-Bromo-Imidazo[5,1-B]Thiazole-7-Carboxylic Acid Ethyl Ester
- CAS Number:901122-44-9
- Molecular formula:C8H7BrN2O2S
- Molecular Weight:275.12

4175-78-4
276 suppliers
$10.00/1g
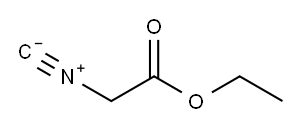
2999-46-4
217 suppliers
$13.43/1gm:
![2-Bromo-Imidazo[5,1-B]Thiazole-7-Carboxylic Acid Ethyl Ester](/CAS/20180629/GIF/901122-44-9.gif)
901122-44-9
19 suppliers
inquiry
Yield:901122-44-9 91.9%
Reaction Conditions:
Stage #1: Ethyl isocyanoacetatewith sodium hydride in N,N-dimethyl-formamide at -10 - 5; for 2 h;
Stage #2: 2,5-dibromo-1,3-thiazole in N,N-dimethyl-formamide at -20 - -5; for 2.33333 h;Product distribution / selectivity;
Steps:
3
Example 3; A suspension of sodium hydride (60% in mineral oil, 13.6 g, 368 mmol) in N,N-dimethylformamide (160 ml) was cooled to -10°C or below under a nitrogen atmosphere. Ethyl isocyanoacetate (36.2 g, 320 mmol) was gradually added dropwise to the cooled suspension, and the mixture was stirred at -3 to 5°C for 2 h. This solution was cooled to -20°C or below and was added dropwise to a solution of 2,5-dibromothiazole (38.8 g, 160 mmol) in N,N-dimethylformamide over a period of about 20 min, and the mixture was stirred at -5 to -20°C for 2 h. Water (8 ml) was added thereto to stop the reaction, and the reaction solution was added to 15 wt% brine (960 ml) under ice cooling. Subsequently, the mixture was adjusted to pH 6 to 7 by the addition of 1 M hydrochloric acid. Subsequently, common salt (102 g) was added thereto at the same temperature, and the mixture was stirred overnight. The resultant precipitate was collected by filtration, was washed with water (136 ml), and was dried under reduced pressure to give ethyl 2-bromoimidazo[5,1-b]thiazole-7-carboxylate (40.4 g, yield 91.9%).
References:
EP1840129,2007,A1 Location in patent:Page/Page column 12