
4-PYRROLIDIN-1-YL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-PYRROLIDIN-1-YL-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:902837-26-7
- Molecular formula:C14H26N2O2
- Molecular Weight:254.37
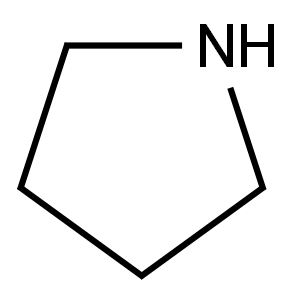
123-75-1
537 suppliers
$10.00/5g

79099-07-3
543 suppliers
$5.00/5g

902837-26-7
25 suppliers
$304.00/1g
Yield:902837-26-7 79%
Reaction Conditions:
Stage #1: pyrrolidine;N-tert-butyloxycarbonylpiperidin-4-onewith sodium acetate in tetrahydrofuran;acetic acid at 20; for 1 h;
Stage #2: with sodium tetrahydroborate;sodium acetate in tetrahydrofuran;acetic acid; for 16 h;
Steps:
10
The white crystalline compound (4.5 g, 0.0226 mol, 1.0 eq.), sodium acetate (2.8 g, 0.034 mol, 1.5 eq.), acetic acid (2.2 ml) and pyrrolidine (2.2 g, 0.031 mol, 1.4 eq) was dissolved in tetrahydrofuran (100 mL). after stirring for 1 hour at room temperature, was added thereto boron hydride, sodium acetate (9.6 g, 0.0452 mol, 2.0 eq.). After 16 hours of reaction, concentrated under reduced pressure, adjusted to alkaline with aqueous sodium hydroxide, and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to give a colorless oily liquid 4-pyrrolidinylpiperidine-1-carboxylic acid tert-butyl ester (4.5 g, yield: 79%).
References:
CN105622638,2016,A Location in patent:Paragraph 0209

24424-99-5
823 suppliers
$13.50/25G

902837-26-7
25 suppliers
$304.00/1g
40064-34-4
366 suppliers
$10.00/5g

902837-26-7
25 suppliers
$304.00/1g

24424-99-5
823 suppliers
$13.50/25G

5004-07-9
132 suppliers
$29.00/1g

902837-26-7
25 suppliers
$304.00/1g