
N-[(2-methyl-5-benzoxazolyl)methyl]carbamic acid 1,1-dimethylethyl ester synthesis
- Product Name:N-[(2-methyl-5-benzoxazolyl)methyl]carbamic acid 1,1-dimethylethyl ester
- CAS Number:903556-81-0
- Molecular formula:C14H18N2O3
- Molecular Weight:262.3
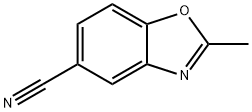
903556-80-9
21 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G
![N-[(2-methyl-5-benzoxazolyl)methyl]carbamic acid 1,1-dimethylethyl ester](/CAS/GIF/903556-81-0.gif)
903556-81-0
12 suppliers
$272.16/1gm:
Yield:-
Reaction Conditions:
Stage #1: 2-methylbenzo[d]oxazole-5-carbonitrile;di-tert-butyl dicarbonatewith sodium tetrahydroborate;nickel dichloride in methanol at 0; for 14 h;
Stage #2: with 3-azapentane-1,5-diamine in methanol;
Stage #3: with citric acid;sodium hydrogencarbonate in water;ethyl acetate;
Steps:
B; 2037.B
The intermediate from step A above (372 mg), di-tert-butyl dicarbonate (1.02 g) and nickel(II) chloride hexahydrate (56 mg) were dissolved in dry methanol (25 mL) and cooled to 0° C. Then sodium borohydride (400 mg) was added in portions and the ice bath removed. The mixture was vigorously stirred for 14 h, then diethylenetriamine (300 μL) was added and the mixture was concentrated to dryness. The residue was diluted with ethyl acetate, washed with 10% citric acid, saturated sodium hydrogen carbonate and brine, dried (MgSO4), concentrated and purified by column chromatography (silica, cyclohexane/EtOAc, 7:3 to 6:4) to afford the intermediate (413 mg) as a colourless oil.
References:
US2006/173183,2006,A1 Location in patent:Page/Page column 98