
10H-Phenothiazine, 10-[(3,4-dihydro-6,7-dimethoxy-1-methyl-2(1H)-isoquinolinyl)carbonyl]- synthesis
- Product Name:10H-Phenothiazine, 10-[(3,4-dihydro-6,7-dimethoxy-1-methyl-2(1H)-isoquinolinyl)carbonyl]-
- CAS Number:904318-41-8
- Molecular formula:C25H24N2O3S
- Molecular Weight:432.53
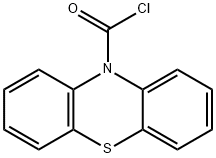
18956-87-1
83 suppliers
$6.00/1g

63283-42-1
25 suppliers
$60.09/250mgs:
![10H-Phenothiazine, 10-[(3,4-dihydro-6,7-dimethoxy-1-methyl-2(1H)-isoquinolinyl)carbonyl]-](/CAS/20210111/GIF/904318-41-8.gif)
904318-41-8
0 suppliers
inquiry
Yield:904318-41-8 72%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 14 h;
Steps:
74
To a stirred solution of 6,7-dimethoxy-l-methyl-l,2,3,4-tetrahydroisoquinoline hydrochloride (70mg, 0.29mmol) in dichloromethane (5mL) was added EPO
References:
WO2006/82354,2006,A1 Location in patent:Page/Page column 37; 38