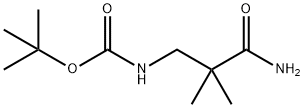
Carbamic acid, N-(3-amino-2,2-dimethyl-3-oxopropyl)-, 1,1-dimethylethyl ester synthesis
- Product Name:Carbamic acid, N-(3-amino-2,2-dimethyl-3-oxopropyl)-, 1,1-dimethylethyl ester
- CAS Number:905831-26-7
- Molecular formula:C10H20N2O3
- Molecular Weight:216.28
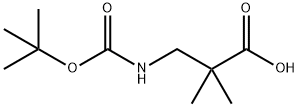
180181-02-6
71 suppliers
$19.00/100mg

905831-26-7
2 suppliers
inquiry
Yield:905831-26-7 71%
Reaction Conditions:
Stage #1: 3-(tert-Butoxycarbonyl)amino-2,2-dimethylpropanoic Acidwith triethylamine in tetrahydrofuran at 0; for 0.333333 h;
Stage #2: with isobutyl chloroformate in tetrahydrofuran at 0; for 1 h;
Stage #3: with ammonium hydroxide in tetrahydrofuran at 0 - 20; for 22 h;
Steps:
tertbutyl N-(2- carbamoyl-2,2-dimethylethyl)carbamate (363)
TEA (5.73 ml, 41 .08 mmol) was added to an ice-cooled (0°C) solution of 3-{[(tert-butoxy)carbonyl]amino}- 2,2-dimethylpropanoic acid (361 ) (5.25 g , 24.16 mmol) in THF (50 ml). The reaction was stirred for 20 min, before the addition of 2-methylpropyl carbonochloridate (4.7 ml, 36.3 mmol) dropwise at 0°C. The reaction was stirred for 1 h before the dropwise addition of 35% NH3 (aqueous solution) (7.48 ml, 135.31 mmol).The reaction mixture was warmed to room temperature and stirred for22 h. Saturated NaHC03 (100 ml) was added and the aqueous layer extracted with DCM (3 x 150 ml). The combined organic layers were dried (MgSC), filtered and evaporated to give an off-white semi-solid (6.61 g). Purification by flash column chromatography (eluting with a gradient of 0-10% MeOH / DCM) gave the title compound (3.7 g, 71 %) as a white solid . 1 H-NMR (CDCI3, 250 MHz): d[ppm]= 5.95 (s, 1 H), 5.30 (s, 1 H), 5.08 (s, 1 H), 3.25 (d , J = 6.6 Hz, 2H), 1 .43 (s, 9H), 1 .21 (s, 6H) HPLCMS (Method A): [m/z]: 239.10 [M+Na]+
References:
WO2017/68089,2017,A2 Location in patent:Page/Page column 322