
(αS,2S)-2-Carboxy-α-[[(1,1-diMethylethoxy)carbonyl]aMino]-1-azetidinebutanoic Acid synthesis
- Product Name:(αS,2S)-2-Carboxy-α-[[(1,1-diMethylethoxy)carbonyl]aMino]-1-azetidinebutanoic Acid
- CAS Number:90599-96-5
- Molecular formula:C13H22N2O6
- Molecular Weight:302.33
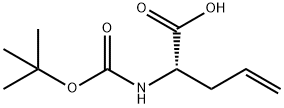
90600-20-7
135 suppliers
$15.00/100mg
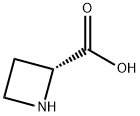
7729-30-8
165 suppliers
$50.00/1g
![(αS,2S)-2-Carboxy-α-[[(1,1-diMethylethoxy)carbonyl]aMino]-1-azetidinebutanoic Acid](/CAS/GIF/90599-96-5.gif)
90599-96-5
8 suppliers
$165.00/50mg
Yield:90599-96-5 93%
Reaction Conditions:
Stage #1: (2S)-2-[(tert-butoxycarbonyl)amino]pent-4-enoic acidwith ozone in methanol at -78;
Stage #2: (2S)-azetidine-2-carboxylic acidwith sodium cyanoborohydride in methanol at 20; for 1 h;Product distribution / selectivity;
Steps:
4
1.6g (7.4 mmol) of Boc-L-allylglycine was dissolved in methanol (25 ml), followed by bubbling with ozone gas at -78 °C. After bubbling until color of the solution turned blue, oxygen was bubbled until the blue color disappeared. After returning to room temperature, 752 mg (7.4 mmol) of L-azetidine-2-carboxylic acid and 470 mg (7.4 mmol) of sodium cyanoborohydride were added, followed by stirring for one hour. After vacuum concentration of the reaction mixture, 2.1 g of compound (3-3) (yield 93%) was obtained by short-pass silica gel chromatography with silica gel (after removing the residue of sodium cyanoborohydride and a small amount of byproduct derived from L-allylglycine with a mobile phase: a mixture solution of ethyl acetate: methanol = 5:1 (v/v), methanol solution was passed through).
References:
EP2103599,2009,A1 Location in patent:Page/Page column 11