
6,6-dimethyl-3-(trifluoromethyl)-6,7-dihydro-1H-indazol-4(5H)-one synthesis
- Product Name:6,6-dimethyl-3-(trifluoromethyl)-6,7-dihydro-1H-indazol-4(5H)-one
- CAS Number:908111-34-2
- Molecular formula:C10H11F3N2O
- Molecular Weight:232.2
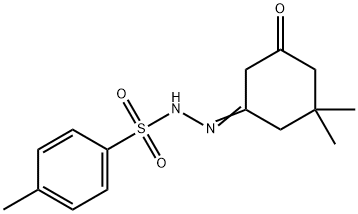
41189-09-7
11 suppliers
inquiry
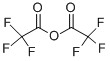
407-25-0
442 suppliers
$9.00/1g

908111-34-2
35 suppliers
$333.69/500MG
Yield:908111-34-2 55%
Reaction Conditions:
with triethylamine in tetrahydrofuran at 55; for 3 h;
Steps:
6.2.11. 6,6-Dimethyl-3-(trifluoromethyl)-6,7-dihydro-1H-indazol-4(5H)-one (15)
To 5.0 g (16.2 mmol) of 14 in THF (90 mL) and Et3N (30 mL) was added trifluoroacetic anhydride (3.4 g, 2.25 mL, 16.2 mmol) in one portion. The resulting red solution was heated at 55 °C for 3 h. After cooling to rt, methanol (8 mL) and 1 M NaOH (8 mL) were added and the solution was stirred for 3 h at rt. The reaction mixture was diluted with 25 mL of saturated NH4Cl, poured into a seperatory funnel and extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with brine (3 × 50 mL), dried over Na2SO4 and concentrated under reduced pressure to give a red oily residue which was chromatographed (hexane/EtOAc, 80:20-60:40) to give 2.08 g (55%) of 15 as an orange solid. TLC (hexane/EtOAc, 6:4) Rf = 0.37; 1H NMR (CDCl3) δ 2.80 (s, 2H), 2.46 (s, 2H), 1.16 (s, 6H); MS (ESI): m/z 231.0 [M-H]-.
References:
Taldone, Tony;Zatorska, Danuta;Patel, Pallav D.;Zong, Hongliang;Rodina, Anna;Ahn, James H.;Moulick, Kamalika;Guzman, Monica L.;Chiosis, Gabriela [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 8,p. 2603 - 2614] Location in patent:experimental part

41189-09-7
11 suppliers
inquiry

407-25-0
442 suppliers
$9.00/1g

908111-34-2
35 suppliers
$333.69/500MG
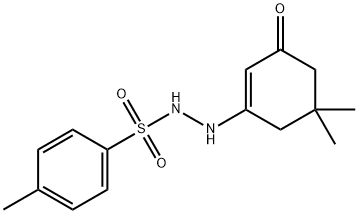
105577-48-8
7 suppliers
inquiry

407-25-0
442 suppliers
$9.00/1g

908111-34-2
35 suppliers
$333.69/500MG
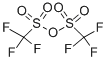
358-23-6
584 suppliers
$10.00/1g

41189-09-7
11 suppliers
inquiry

908111-34-2
35 suppliers
$333.69/500MG
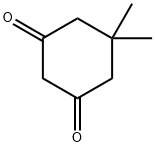
126-81-8
408 suppliers
$5.00/10g

908111-34-2
35 suppliers
$333.69/500MG