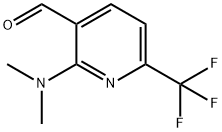
3-Pyridinecarboxaldehyde, 2-(dimethylamino)-6-(trifluoromethyl)- synthesis
- Product Name:3-Pyridinecarboxaldehyde, 2-(dimethylamino)-6-(trifluoromethyl)-
- CAS Number:910487-56-8
- Molecular formula:C9H9F3N2O
- Molecular Weight:218.18
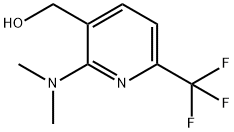
910487-55-7
0 suppliers
inquiry

910487-56-8
4 suppliers
inquiry
Yield:910487-56-8 83%
Reaction Conditions:
Stage #1: [2-(dimethylamino)-6-(trifluoromethyl)pyridin-3-yl]methanolwith oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 0.5 h;
Stage #2: with triethylamine in dichloromethane at -78 - 20; for 1.5 h;
Steps:
79.79C 2-[2-(Dimethylamino)-6-(trifluoromethyl)pyridin-3-yl]-N-((1R)-1-{2-fluoro-5-methyl-4-[(methylsulfonyl)amino]phenyl}ethyl)cyclopropanecarboxamide
79C) 2-(Dimethylamino)-6-(trifluoromethyl)nicotinaldehyde To a DCM (17 ml) solution of ethanedioyl dichloride (1.5 ml, 11.4 ml) was added dimethyl sulfoxide (1.3 ml, 17.2 mmol) at -78° C. and the mixture was stirred for 15 minutes at -78° C. Then to the mixture was slowly added a DCM solution of the compound of Example 79B (1.3 g, 5.7 mmol) at -78° C. and the mixture was stirred for 30 minutes followed by addition of triethylamine (5.8 ml, 57.2 mmol) and stirring for 30 minutes at -78° C. The reaction temperature was allowed to warm to room temperature and stirred for 1 hour. Then the reaction was quenched with water and extracted with EtOAc, dried over magnesium sulfate, and the solvent evaporated. The crude residue was purified by silica gel column chromatography, eluding with hexane/EtOAc (7:1), to give the title compound (1.0 g, 83%). 1H NMR (300 MHz, CDCl3) δ 3.14-3.19 (6H, m), 7.02-7.11 (1H, m), 8.03-8.12 (1H, m), 8.03-8.12 (1H, m), 9.97-10.0 (1H, m).
References:
US2006/211741,2006,A1 Location in patent:Page/Page column 75
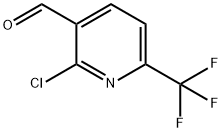
944900-06-5
65 suppliers
$65.00/100mg
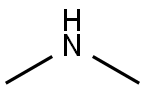
124-40-3
546 suppliers
$18.00/100ml

910487-56-8
4 suppliers
inquiry