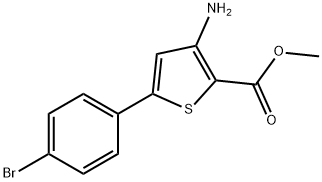
3-AMINO-5-(4-BROMOPHENYL)THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:3-AMINO-5-(4-BROMOPHENYL)THIOPHENE-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:91076-95-8
- Molecular formula:C12H10BrNO2S
- Molecular Weight:312.18

78583-87-6
17 suppliers
inquiry

2365-48-2
342 suppliers
$14.00/25g

91076-95-8
19 suppliers
$95.00/1g
Yield:91076-95-8 72%
Reaction Conditions:
with sodium methylate in methanol; for 3 h;Reflux;
Steps:
General procedure for the preparation of 3-aminothiophene-2-carboxylates (1a-o)
General procedure: An apropriate substrate S2a-n, from the previous step, in dry MeOH (15 ml) [THF (15 ml) was used in the case of 3,4-dirnethoxyphenyl substrate S2m] was added dropwise to a stirred mixture of methyl thioglycolate (3.5 ml, 38 mmol) and NaOMe, prepared by dissolving Na (1.15 g, 50 mmol) in dry MeOH (20 ml), at 0 °C during 30 min. The formed suspension was intensely stirred and heated at reflux for 3 h. Then, the reaction mixture was poured in ice cold water (100 ml) and neutralized with AcOH (1.10 ml). The precipitate was filtered, washed with water (5x10 ml), MeOH (10 ml) and dried under air. Esters 1 were purified by crystallization from EtOH, except for compoimd 1l, which was crystallized from Et2O. Yields of esters 1a-n are based on methyl ketones S1a-n. 5-Styryl-substituted derivative 1o was prepared in the same manner by the reaction of substrate S2o (4.55 g, 24 mmol) with methyl thioglycolate (2.75 ml, 30 mmol) in the presence of NaOMe, from Na (0.92 g, 40 mmol), dry MeOH (30 ml). Compound 1o was purified by crystallization from EtOH.
References:
Irgashev, Roman A.;Steparuk, Alexander S.;Rusinov, Gennady L. [Tetrahedron Letters,2019,vol. 60,# 43,art. no. 151185] Location in patent:supporting information

99-90-1
467 suppliers
$6.00/25g

91076-95-8
19 suppliers
$95.00/1g