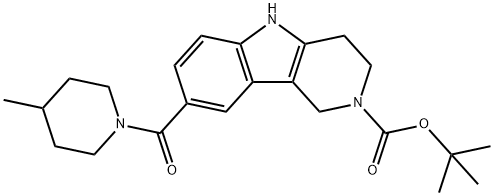
tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate synthesis
- Product Name:tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate
- CAS Number:910797-04-5
- Molecular formula:C23H31N3O3
- Molecular Weight:397.51
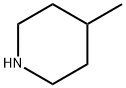
626-58-4
198 suppliers
$10.00/1g
![1,3,4,5-TETRAHYDRO-PYRIDO[4,3-B]INDOLE-2,8-DICARBOXYLIC ACID 2-TERT-BUTYL ESTER](/CAS/GIF/300715-96-2.gif)
300715-96-2
29 suppliers
$117.00/100mg
![tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20150408/GIF/910797-04-5.gif)
910797-04-5
10 suppliers
inquiry
Yield:910797-04-5 94.5%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;HATU in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
1.B
The product from Step A (2-(tert-butoxycarbonyl)-2,3,4,5-tetrahydro-1H- pyrido[4,3-b]indole-8-carboxylic acid, 4.80 g, 15.2 mmol) was dissolved in DMF (80 mL), 4-methylpiperidine (2 mL, 16.9 mmol), diisopropylethylamine (7.0 mL, 38.9 mmol), and HATU (7.60 g, 20.0 mmol) was added successively. The mixture was stirred at room temperature for 1 h, and the reaction was quenched with water (5 mL). The solvent was removed in vacuo to give a light yellow solid, and the solid was treated with water (100 mL) and collected by filtration, then recrystallized in methanol to afford the desired product (4.60 g), the filtrate was concentrated in vacuo and purified on silica gel (0-40% EtOAc in dichloromethane) to give 1.10 g additional desired product, the combined yield was 94.5%. MS: 398.1 (M+l).
References:
WO2006/101434,2006,A1 Location in patent:Page/Page column 31
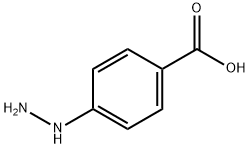
619-67-0
413 suppliers
$6.00/5g
![tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20150408/GIF/910797-04-5.gif)
910797-04-5
10 suppliers
inquiry

24424-99-5
825 suppliers
$13.50/25G
![tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20150408/GIF/910797-04-5.gif)
910797-04-5
10 suppliers
inquiry
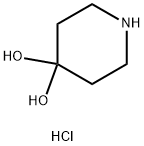
40064-34-4
366 suppliers
$10.00/5g
![tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20150408/GIF/910797-04-5.gif)
910797-04-5
10 suppliers
inquiry
![2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indole-8-carboxylic acid](/CAS/GIF/929345-60-8.gif)
929345-60-8
16 suppliers
inquiry
![tert-butyl 8-[(4-Methylpiperidin-1-yl)carbonyl]-1,3,4,5-tetrahydro-2H-pyrido[4,3-b]indole-2-carboxylate](/CAS/20150408/GIF/910797-04-5.gif)
910797-04-5
10 suppliers
inquiry