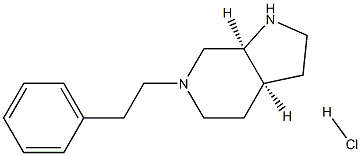
(3aS,7aS)-6-Phenethyloctahydro-1H-pyrrolo[2,3-c]pyridine hydrochloride synthesis
- Product Name:(3aS,7aS)-6-Phenethyloctahydro-1H-pyrrolo[2,3-c]pyridine hydrochloride
- CAS Number:912338-15-9
- Molecular formula:C15H23ClN2
- Molecular Weight:266.8095
![1H-Pyrrolo[2,3-c]pyridine, octahydro-1-[(1R)-1-(1-naphthalenyl)ethyl]-6-(2-phenylethyl)-, (3aR,7aS)-](/CAS/20210305/GIF/912338-20-6.gif)
912338-20-6
0 suppliers
inquiry
![(3aS,7aS)-6-Phenethyloctahydro-1H-pyrrolo[2,3-c]pyridine hydrochloride](/CAS/20180601/GIF/912338-15-9.gif)
912338-15-9
6 suppliers
inquiry
Yield:912338-15-9 82%
Reaction Conditions:
with hydrogen;palladium(II) hydroxide/carbon in methanol; under 2585.81 Torr; for 16 h;Parr bottle;
Steps:
5; 208.6
Into a 2.5-liter Parr bottle are charged 20% Pd(OH)2 on carbon (11.2 g, 50% wet) under nitrogen atmosphere. A solution of compound A6 (56 g, 0.15 mol) in methanol (1 L) is added. The mixture is hydrogenated at 50 psi for 16 h until all A6 is consumed. The mixture is filtered through a pad of celite under nitrogen atmosphere. The filtrate is concentrated under vacuum at 35° C. to give an oil. The oil containing A7 and the by-product is dissolved into ethyl acetate. A solution of 6N HCl in IPA (27 mL) is diluted with EtOAc (60 mL) and added to the solution containing A7 over a period of 40 min at 18-27° C. After the addition, the mixture is cooled to 0° C. and stirred for an additional 1 h. Any solid is collected by filtration to obtain A7 hydrochloride salt as a white solid (33 g, 82%): m.p.=172-178° C. 1H NMR (500 MHz, CDCl3) δ 7.12-7.32 (m, 5H), 3.50 (s, 1H), 3.15-3.35 (m, 2H), 2.45-2.95 (m, 8H), 2.20 (s, 2H), 1.95 (m, 1H), 1.70 (m, 2H), 1.45 (s, 1H). By treating the hydrochloride salt with aqueous 1N NaOH solution, the free base of A7 is obtained as an oil: 1H NMR (500 MHz, CDCl3) δ 7.15-7.35 (m, 5H), 3.05 (m, 1H), 2.96 (qAB, J=8.2, 4.0 Hz, 1H), 2.87 (m, 2H), 2.70 (m, 2H), 2.60 (m, 1H), 2.49 (m, 2H), 2.40 (bs, 1H), 2.30 (dd, J=11.6, 3.7 Hz, 1H), 2.02 (td, J=11.0, 3.0 Hz, 1H), 1.88 (m, 1H), 1.77 (m, 1H), 1.52 (m, 2H), 1.36 (m, 1H). 13C NMR (125 Hz, CDCl3) δ 141.0, 129.1, 128.7, 126.3, 60.9, 58.6, 55.0, 53.0, 44.9, 36.6, 34.1, 31.9, 27.8. Rotation: α25Na=-11.5° [c 9.57 mg/1 mL CH3CN].
References:
US2005/234042,2005,A1 Location in patent:Page/Page column 77-78; 80