
tert-Butyl 6-aMino-7-chloro-3,4-dihydroisoquinoline-2(1H)-carboxylate synthesis
- Product Name:tert-Butyl 6-aMino-7-chloro-3,4-dihydroisoquinoline-2(1H)-carboxylate
- CAS Number:912846-75-4
- Molecular formula:C14H19ClN2O2
- Molecular Weight:282.77
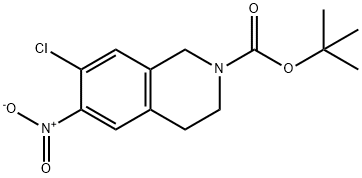
912846-74-3
24 suppliers
$72.00/100mg

912846-75-4
27 suppliers
$445.00/1g
Yield:912846-75-4 62%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;methanol;water at 60; for 4 h;
Steps:
B18.1 Step 1. tert-butyl 6-amino-7-chloro-3,4-dihydroisoquinoline-2(1H)-carboxylate
A solution of tert- butyl 7-chl oro-6-nitro-3,4-dihydroisoquinoline-2(l H)- carboxylate (0.136 g, 0.434 mmol), iron (0.097 g, 1.736 mmol), and ammonium chloride (0.139 g, 2.60 mmol) in THF (0.723 mL)/methanol (0.723 mL)/water (0.723 mL) was stirred at 60 °C for 4 hr. Then, the solution was filtered through Celite and rinsed with ethyl acetate and methanol. The filtrate was washed with water and brine, dried over sodium sulfate, and concentrated under reduced pressure. The crude product was purified by Teledyne ISCO CombiFlash RF+ (0-100% ethyl acetate in hexanes) to provide the desired product as a brown solid (0.0762 g, 0.269 mmol, 62%). LCMS calculated for C14H20CIN2O2 (M+H)+: m/z = 283.1; Found: 283.3.
References:
WO2020/168178,2020,A1 Location in patent:Page/Page column 140-141