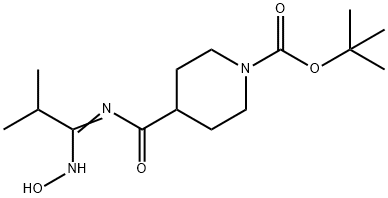
4-(1-Hydroxyimino-2-methylpropylcarbamoyl)-piperidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:4-(1-Hydroxyimino-2-methylpropylcarbamoyl)-piperidine-1-carboxylic acid tert-butyl ester
- CAS Number:913264-41-2
- Molecular formula:C15H27N3O4
- Molecular Weight:313.39
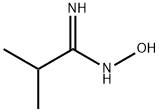
35613-84-4
102 suppliers
$21.75/1g
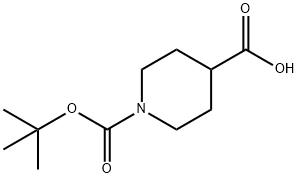
84358-13-4
391 suppliers
$5.00/5g

913264-41-2
12 suppliers
$60.00/100mg
Yield:913264-41-2 73%
Reaction Conditions:
Stage #1: N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acidwith 1,1'-carbonyldiimidazole in dichloromethane at 20; for 1 h;Heating / reflux;
Stage #2: isobutyramide oxime in dichloromethane at 20; for 18 h;
Steps:
15
Λ/,Λ/-Carbonyldiimidazole (24.75 g, 153 mmol) was added portionwise at room temperature to a solution of terf-butoxycarbonyl-isonipecotic acid (35.0 g, 153 mmol) in dichloromethane (400 ml). The reaction mixture was stirred at room temperature for T hour. A solution of the amidoxime of preparation 14 (20.0 g, 200 mmol) in dichloromethane (100 ml) was then added dropwise, after which time the reaction mixture was stirred at room temperature for 18 hours. The reaction mixture was quenched with water (300 ml) and the layers were separated. The organic layer was washed with 1M citric acid (800 ml) and saturated sodium bicarbonate solution (300 ml). The organic layer was dried over magnesium sulfate, filtered and decolourising charcoal was added to the filtrate. The mixture was stirred for 5 minutes, filtered and concentrated under reduced pressure to yield the title compound (35.05 g, 73%) as a solid.1H NMR (400MHz, CDCI3): δ 1.21 (d, 6H), 1.45 (s, 9H), 1.67-1.77 (m, 2H), 1.88-1.94 (m, 2H), 2.55- 2.66 (m, 2H), 2.80-2.86 (m, 2H), 4.03-4.10 (m, 2H), 4.60 (brs, 1 H); LRMS ESI+ m/z 336 [MNa]+.
References:
WO2006/114706,2006,A1 Location in patent:Page/Page column 34