5-Fluoro-7-nitroindole-2-carboxylic acid ethyl ester synthesis
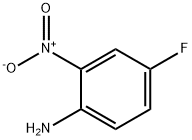
- Product Name:5-Fluoro-7-nitroindole-2-carboxylic acid ethyl ester
- CAS Number:913287-12-4
- Molecular formula:C11H9FN2O4
- Molecular Weight:252.2
Yield:913287-12-4 585 mg
Reaction Conditions:
Stage #1: 2-nitro-4-fluoroanilinewith hydrogenchloride;sodium nitrite in water;
Stage #2: 2-acetylpropanoic acid ethyl esterwith potassium hydroxide in ethanol;water at 0 - 5; for 0.166667 h;
Stage #3: at 75 - 100; for 4 h;
Steps:
6.1
A solution of NaN02 (2.320 g, 33.6 mmol) in water (NaN02) (8.69 ml, 482 mmol) was added dropwise over 20 minutes to 4-fluoro-2-nitroaniline (5 g, 32.0 mmol) in water (aniline) (11.14 ml, 618 mmol) and HCI cone. (13.15 ml, 160 mmol. In a separate containing ethyl 2-methylacetoacetate (4.66 ml, 32.0 mmol) in EtOH (33.1 ml, 567 mmol) cooled to 0-5 °C was added dropwise KOH 45%wt. in water (8.23 ml, 96 mmol) followed by ice-cold water (66.1 ml, 3667 mmol) and It was stirred for 10 minutes. To this solution was added the 1st solution dropwise and stirred overnightAn orange suspension and big black chunks were formed. The reaction mixture was extracted with Et20 (3 x 100 mL) and the combined organic layers were washed with water (2 x 100 mL) then with brine (100 mL). The organic layer was dried over MgS04, filtered and concentrated to dryness to give crude ethyl (E)-ethyl 2-(2-(4-fluoro-2- nitrophenyl)hydrazono)propanoate (7.30 g, 27.1 mmol, 85 % yield). The previous compound in polyphosphoric acid (41.5 ml, 873 mmol) was heated to 110 °C for 1 hour then cooled to 75 °C. After 3 hrs, the mixture was cooled to 20 °C and water (100 mL) was added and stirred for 30 minutes. EA was then added (600 mL) and the aqueous phase was neutralized with sat. NaHC03 (300 mL) (not close to neutral pH). More water was added (500 mL) and then solid NaHC03 (ca. 250 g). More water (500 mL) and EtOAc (500 mL) were added and the mixture was filtered. The solid was washed solids with Water (500 mL) and EtOAc (500 mL). The layers were separated and the organic layer was washed with water (500 mL) and with brine (300 mL). The organic layer was dried over MgSC>4, filtered and concentrated to give 3.42 g as a brown solid. The residue was purified on ISCO using a RediSep Gold column (Hex/EtOAc) . The crude product was dissolved and loaded onto a pre-column (dissolved crude in EtOAc). A second purification was needed to yield ethyl 5-fluoro-7-nitro-1 H-indole-2-carboxyiate (585 mg, 8.55 % yield). 1H NMR (DMSO-d6) δ: 11.59 (br. s., 1 H), 8.18 (dd, J = 9.0, 2.3 Hz, 1 H), 8.12 (dd, J = 8.6, 2.3 Hz, 1 H), 7.44 (s, 1 H), 4.39 (q, J = 7.0 Hz, 2H), 1.36 (t, J = 7.0 Hz, 3H).
References:
WO2019/84662,2019,A1 Location in patent:Page/Page column 192
![Propanoic acid, 2-[2-(4-fluoro-2-nitrophenyl)hydrazinylidene]-, ethyl ester](/CAS/20210305/GIF/1172997-05-5.gif)
1172997-05-5
1 suppliers
inquiry

913287-12-4
15 suppliers
inquiry