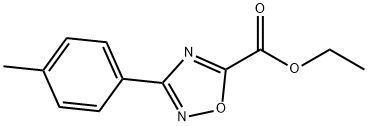
3-(4-methylphenyl)-1,2,4-Oxadiazole-5-carboxylic acid ethyl ester synthesis
- Product Name:3-(4-methylphenyl)-1,2,4-Oxadiazole-5-carboxylic acid ethyl ester
- CAS Number:91393-16-7
- Molecular formula:C12H12N2O3
- Molecular Weight:232.24
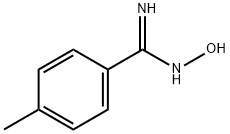
19227-13-5
104 suppliers
$10.00/250mg
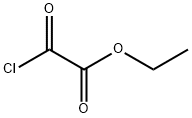
4755-77-5
345 suppliers
$9.00/25g

91393-16-7
20 suppliers
$94.25/1g
Yield:91393-16-7 84.56 %
Reaction Conditions:
with triethylamine in acetonitrile at 72;Cooling with ice;
Steps:
15.2 (2) Preparation of 3-(4-methylphenyl)-1,2,4-oxadiazole-5-carboxylic acid ethyl ester:
The intermediate N-hydroxyl-4-methylbenzamide (5.00g, 33.29mmol),Triethylamine (5.05g, 49.94mmol)and 39.95mL of acetonitrile were added to the three-necked flask,And monoethyl oxalyl chloride (5.54g, 39.95mmol) was added dropwise under ice bath,After continuing to keep the temperature stirring for 0.5h,Heat up to 72°C and reflux for 7 hours;After the reaction was completed, the resulting solid was removed by suction filtration.The solvent was removed from the filtrate under reduced pressure, and the concentrate was diluted with ethyl acetate,and extracted with ethyl acetate, washed with water and saturated brine,The organic phase was dried over anhydrous sodium sulfate,By column chromatography (petroleum ether: ethyl acetate = 30:1)6.37 g of ethyl 3-(4-methylphenyl)-1,2,4-oxadiazole-5-carboxylate was obtained as a white solid,Yield 84.56%.
References:
CN114933573,2022,A Location in patent:Paragraph 0171; 0173-0174

19227-13-5
104 suppliers
$10.00/250mg

4755-77-5
345 suppliers
$9.00/25g

91393-16-7
20 suppliers
$94.25/1g

19227-13-5
104 suppliers
$10.00/250mg

91393-16-7
20 suppliers
$94.25/1g
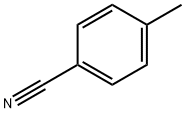
104-85-8
326 suppliers
$11.00/25g

91393-16-7
20 suppliers
$94.25/1g