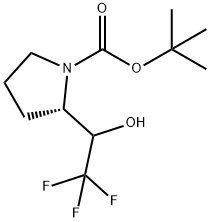
(S)-tert-butyl 2-(2,2,2-trifluoro-1-hydroxyethyl)pyrrolidine-1-carboxylate synthesis
- Product Name:(S)-tert-butyl 2-(2,2,2-trifluoro-1-hydroxyethyl)pyrrolidine-1-carboxylate
- CAS Number:913979-68-7
- Molecular formula:C11H18F3NO3
- Molecular Weight:269.26
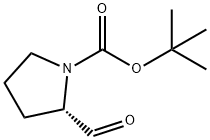
69610-41-9
255 suppliers
$9.00/1g
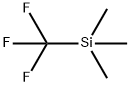
81290-20-2
408 suppliers
$13.00/1g

913979-68-7
6 suppliers
inquiry
![1-Pyrrolidinecarboxylic acid, 2-[2,2,2-trifluoro-1-[(trimethylsilyl)oxy]ethyl]-, 1,1-dimethylethyl ester, (2S)-](/CAS/20210305/GIF/913979-69-8.gif)
913979-69-8
0 suppliers
inquiry
Yield:-
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuran at 0 - 20; for 1 h;
Steps:
130.A
Commercially available 2-formyl-pyrrolidine-l-carboxylic acid tert-butyl ester (330 mg) in anhydrous THF (5 ml) was cooled to 0 °C and trimethyl-trifluoromethylsilane (300 μ) added, followed by addition of tetrabutylammoniumfluoride (60 μl; 1 M in THF). The reaction mixture was allowed to warm to rt and then stirred for 1 h. After dilution with diethyl ether, the organic phase was washed with brine and the aqueous phase extracted with diethyl ether. The combined organic phases were dried (MgSO4) and evaporated to afford the title compounds as a 1:1 mixture of alcohol and TMS ether (490 mg, 97 %, [MH-Boc]+ = 242 (TMS ether); [MH-Bocf = 170 (alcohol)).
References:
WO2006/116157,2006,A2 Location in patent:Page/Page column 135