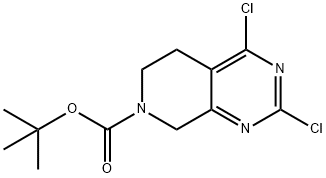
tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate synthesis
- Product Name:tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate
- CAS Number:916420-27-4
- Molecular formula:C12H15Cl2N3O2
- Molecular Weight:304.17

24424-99-5
824 suppliers
$13.50/25G
![2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine HCl salt](/CAS/GIF/1000578-08-4.gif)
1000578-08-4
75 suppliers
$195.00/100mg
![tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate](/CAS/GIF/916420-27-4.gif)
916420-27-4
226 suppliers
$12.00/100mg
Yield:916420-27-4 74%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 16 h;
Steps:
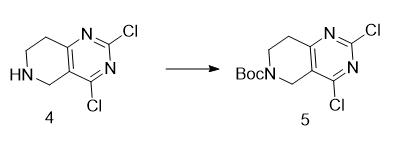
4.4
To a 0 °C solution of the intermediate 4 (270 mg, 1.33 mmol) in DCM (30 mL) were added (Boc)20 (348 mg, 1.6 mmol) and TEA (200 mg, 2.0 mmol). The resulting solution was stirred at the room temperature for 16 hours. It was then diluted with treated water (30 mL) and DCM (30 mL), the layers were separated and the aqueous phase was extracted with DCM (30 mL x 2). The combined organic layers were washed with brine, dried over sodium sulfate. The Na2S04 was removed by filtration, and the volatiles were removed under reduced pressure. The resulting residue was purified by flash chromatography using a mixture of hexane and ethyl acetate to provide the product 5 (300 mg, 74%). LRMS (M + H+) m/z: calcd 305.17; found 305.24. 1H-NMR (300 MHz, CDCls) δ 4.624 (s, 2H), 3.739-3.700 (t, J=5.9 Hz, 2H), 2.848-2.811 (t, j=5.6 Hz, 2H), 1.472 (s, 9H).
References:
ZHOU, Han-Jie;PARLATI, Francesco;WUSTROW, David WO2014/15291, 2014, A1 Location in patent:Page/Page column 174; 175
39514-19-7
137 suppliers
$17.00/1g
![tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate](/CAS/GIF/916420-27-4.gif)
916420-27-4
226 suppliers
$12.00/100mg
![7-BENZYL-5,6,7,8-TETRAHYDROPYRIDO[3,4-D]PYRIMIDINE-2,4(1H,3H)-DIONE](/CAS/GIF/62459-02-3.gif)
62459-02-3
113 suppliers
$56.00/25 mg
![tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate](/CAS/GIF/916420-27-4.gif)
916420-27-4
226 suppliers
$12.00/100mg
![Pyrido[3,4-d]pyrimidine, 2,4-dichloro-5,6,7,8-tetrahydro-7-(phenylmethyl)-](/CAS/GIF/1059735-34-0.gif)
1059735-34-0
149 suppliers
$32.00/250mg
![tert-Butyl 2,4-dichloro-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidine-7-carboxylate](/CAS/GIF/916420-27-4.gif)
916420-27-4
226 suppliers
$12.00/100mg