
[2-Amino-5-(methyloxy)phenyl](4-chlorophenyl)methanone synthesis
- Product Name:[2-Amino-5-(methyloxy)phenyl](4-chlorophenyl)methanone
- CAS Number:91713-54-1
- Molecular formula:C14H12ClNO2
- Molecular Weight:261.7

38527-50-3
9 suppliers
inquiry
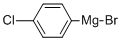
873-77-8
145 suppliers
$61.21/100ml
methanone](/CAS/20150408/GIF/91713-54-1.gif)
91713-54-1
21 suppliers
$165.00/500mg
Yield:91713-54-1 88%
Reaction Conditions:
Stage #1: 6-methoxy-2-methyl-4H-benzo[d][1,3]oxazin-4-one;4-chlorophenyl-magnesium bromide in diethyl ether;toluene at 0 - 20; for 1 h;
Stage #2: with hydrogenchloride;lithium hydroxide monohydrate in ethanol; for 2 h;Reflux;
Stage #3: with sodium hydroxide in ethyl acetate;
Steps:
79
Intermediate 79: [2-amino-5-(methyloxy)phenyll(4-chlorophenyl)methanone To a solution of Intermediate 83 (40.0 g, 0.21 mol, 1 equiv.) in a toluene/ether (2/1 ) mixture (760 mL) at 0°C was added dropwise a solution of 4-chlorophenylmagnesium bromide (170 mL, 1 M in Et20, 0.17 mol, 0.8 equiv.). The reaction mixture was allowed to warm to RT and stirred for 1 h before being quenched with 1 N HCI (200 mL). The aqueous layer was extracted with EtOAc (3 x 150 mL) and the combined organics were washed with brine (100 mL), dried over Na2S04, filtered and concentrated under reduced pressure. The crude compound was then dissolved in EtOH (400 mL) and 6N HCI (160 mL) was added. The reaction mixture was refluxed for 2 h before being concentrated to one-third in volume. The resulting solid was filtered and washed twice with ether before being suspended in EtOAc and neutralised with 1 N NaOH. The aqueous layer was extracted with EtOAc (3 x 150 mL) and the combined organics were washed with brine (150 mL), dried over Na2S04, filtered and concentrated under reduced pressure. The title compound was obtained as a yellow solid (39 g, 88 % yield); LC/MS: m/z 262 [M+H]+, Rt 2.57 min.
References:
WO2011/54844,2011,A1 Location in patent:Page/Page column 84
214971-09-2
2 suppliers
inquiry
106-39-8
443 suppliers
$14.00/5g
methanone](/CAS/20150408/GIF/91713-54-1.gif)
91713-54-1
21 suppliers
$165.00/500mg
6705-03-9
256 suppliers
$9.00/1g
methanone](/CAS/20150408/GIF/91713-54-1.gif)
91713-54-1
21 suppliers
$165.00/500mg

37795-77-0
100 suppliers
$11.00/250mg
methanone](/CAS/20150408/GIF/91713-54-1.gif)
91713-54-1
21 suppliers
$165.00/500mg

38527-50-3
9 suppliers
inquiry
methanone](/CAS/20150408/GIF/91713-54-1.gif)
91713-54-1
21 suppliers
$165.00/500mg