
1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-, (1S,4R)- synthesis
- Product Name:1-Naphthalenamine, 4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-, (1S,4R)-
- CAS Number:91797-60-3
- Molecular formula:C17H17Cl2N
- Molecular Weight:306.23
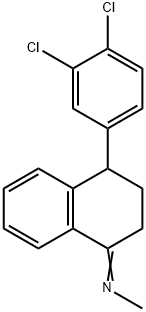
79560-20-6
108 suppliers
inquiry
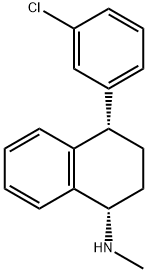
871838-58-3
15 suppliers
$185.00/1mg
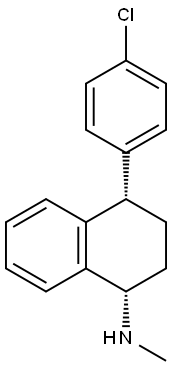
91797-63-6
3 suppliers
inquiry
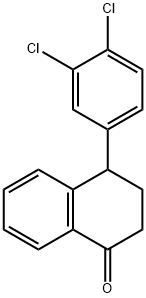
79560-19-3
276 suppliers
$6.00/5g

91797-60-3
1 suppliers
inquiry

79617-96-2
155 suppliers
inquiry
Yield:79617-96-2 87 - 91 %Chromat. ,91797-60-3 7 - 9.4 %Chromat. ,79560-19-3 0.2 - 0.7 %Chromat.
Reaction Conditions:
with hydrogen;SA-3135 support, Co(NO3)2, H2O; dried and calcined ones or twice (Co 11 - 25percent); reduction with H2 in tetrahydrofuran at 120 - 150; under 6000.6 Torr;
Steps:
1-4 Example 1: Preparation of the Proprietary Cobalt Catalyst
The catalyst was prepared using the incipient wetness impregnation of alumina-silica. The support precursor catalyst reduction purchased from Saint-Gobain NORPRO (Stow, Ohio) was first calcined at 500°C for 12 hours before impregnation. Characteristics of the support SA3 135 is given in Table 5. Calcined SA-3 135 (5 grams), granules 1-1.2 mm, was charged into glass flask and evaporated at P=0. 02 bar and 25°C over 1 hour. An aqueous solution of cobalt nitrate (4.0 ml) containing 4.36 g OF CO (N03) 2X 6H20 (98%), purchased from Aldrich (MILWAUKEE, Wisconsin), was added dropwise so that surface of all the granules was saturated by the salt solution. The catalyst was dried at 120°C for 4 hours and calcined at 500°C for 4 hours. The Energy Dispersive X-Ray ("EDX") analysis indicated the contents of Co, AL, Si, 0: 15.0, 35.8, 13.8 and 35.4 % wt. , respectively. The XRD patterns of the material were evident for the existence of the phase Co3O4 : (peaks at 28 : 18.96, 31.24, 36. 68, 38,78, 44.75, 57.55). The average crystal size of CoO estimated in XRD study was 50 nm. The catalyst had surface area L LM2/G, with an average pore diameter 183 angstroms. The catalyst (2.3g) was loaded into a tubular stainless steal reactor (6 mm ID and 150mm length). In the stream of hydrogen at GHSV (gas hour space velocity) 2500h-1, the temperature was gradually ramped to 500°C, (5°C/MIN), and then the temperature was maintained constant at 500°C for 4 hours. The operating conditions of catalyst reduction were selected over Temperature Programmed Reduction ("TPR") studies carried out in AMI-100 Catalyst Characterization System from Zeton-Altamira (Pittsburgh, Pennsylvania). The XRD analysis of the reduced catalysts indicates the existence of the phase Co and CoO (peaks at 29 : 36.8, 42.47, 61.41, 73.69). After the completion of the catalyst reduction, the temperature in the reactor was decreased to 120°C, and the total pressure of hydrogen was increased to 8 bar. A solution containing N-methyl4 (3, 4-dichlorophenyl)-1-(-2H)- naphtalenimine (Imine) in TETRAHYDROFURAN (THF) S PURCHASED FROM BIOLAB (99. 5%), HAVING A concentration of 30 g/L was then fed with hydrogen to the reactor. The rate of the solution feed was 25g/h and the rate of hydrogen feed was 150 ml/mm. (The products were analyzed by GC HP6890 (Palo Alto, California): capillary column DB-17, 30 m x 0.53 mm x LU.. The initial temperature was 160°C for 26 minutes, and then the temperature was increased at a rate OF 30°C/MIN to 250°C). The results of the GC analysis under conditions mentioned above are shown in table 7, which indicates that the imine was completely hydrogenated to a mixture containing: cis- (1S,4S)-N-Methyl-(3,4-dichlorophenyl)-1, 2,3, 4-tetrahydro-1-naphtalenamine (cis-Sertraline) as the major component, TRANS- (1S, 4R) -N-Methyl-4 (3,4-dichlorophenyl)- 1, 2,3, 4-. TETRAHYDRO-1-NAPHTALENAMINE (trans- Sertraline), (LYS, 4S) -N-Methyl-4 (3-DICHLOROPHENYL)-1, 2,3, 4-tetrahydro-1-naphtalenamine (DCS-1), (1 S, 4S)-N-Methyl-4- (4-dichlorophenyl)-1, 2,3, 4-TETRAHYDRO-1-NAPHTALENAMINE (DCS-2) and SERTRALONE. The content of cis-sertraline in the product of imine hydrogenation was 91%.
References:
WO2004/92110,2004,A2 Location in patent:Page 19-22

79617-96-2
155 suppliers
inquiry

79617-98-4
24 suppliers
$496.00/1mg

91797-60-3
1 suppliers
inquiry
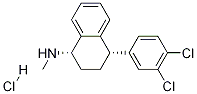
79951-46-5
2 suppliers
inquiry

79560-20-6
108 suppliers
inquiry

79617-98-4
24 suppliers
$496.00/1mg

91797-60-3
1 suppliers
inquiry
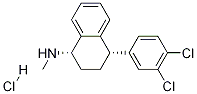
79951-46-5
2 suppliers
inquiry

79617-96-2
155 suppliers
inquiry
![Methanamine, N-[(4R)-4-(3,4-dichlorophenyl)-3,4-dihydro-1(2H)-naphthalenylidene]-](/CAS/20210305/GIF/312620-95-4.gif)