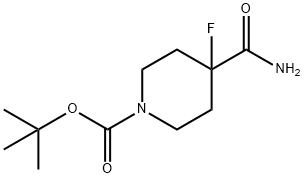
tert-Butyl 4-carbamoyl-4-fluoropiperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-carbamoyl-4-fluoropiperidine-1-carboxylate
- CAS Number:918431-92-2
- Molecular formula:C11H19FN2O3
- Molecular Weight:246.28
614731-04-3
78 suppliers
$33.00/100mg

918431-92-2
9 suppliers
inquiry
Yield:918431-92-2 100%
Reaction Conditions:
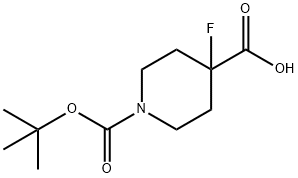
Stage #1: 1-[(tert-butoxy)carbonyl]-4-fluoropiperidine-4-carboxylic acidwith 4-methyl-morpholine;isopropyl chloroformate in 1,2-dimethoxyethane;toluene at -15; for 0.166667 h;
Stage #2: with ammonia in 1,4-dioxane at 20; for 18 h;
Steps:
4 4. Preparation of 4-carbamoyl-4-fluoro-piperidine-1-carboxylic acid tert-butyl ester
A solution of 1-boc-4-fluoro-4-piperidinecarboxylic acid (300 mg, 1.15 mmol) in ethylene glycol dimethyl ether (15 mL) was treated with 4-methylmorpholine (0.13 mL, 1.15 mmol) and isopropyl chloroformate (1 M solution in toluene, 1.38 mL, 1.38 mmol) at -15°C. After stirring for 10 mm, ammonia solution (0.5 M in dioxane, 3.50 mL, 1.75 mmol) was added.The reaction mixture was stirred at RT for 18h. The solvents were evaporated under reduced pressure, the crude product was dissolved in EtOAc, washed with 1 N NaOH solution, water and brine and the organic layers were dried over sodium sulfate, filtered and evaporated to dryness to get 185 mg (65 %) of a white powder. The product was used for the next step without further purification. LCIMS (Method A): Rt = 1.79 mm,(M+H) 173.
References:
WO2015/144290,2015,A1 Location in patent:Page/Page column 82

416852-82-9
78 suppliers
$42.00/100mg

918431-92-2
9 suppliers
inquiry