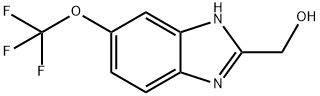
2-(Hydroxymethyl)-5-(trifluoromethoxy)benzimidazole synthesis
- Product Name:2-(Hydroxymethyl)-5-(trifluoromethoxy)benzimidazole
- CAS Number:919054-04-9
- Molecular formula:C9H7F3N2O2
- Molecular Weight:232.16
Yield: 75%
Reaction Conditions:
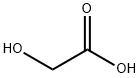
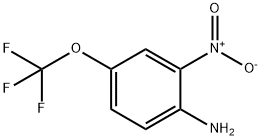
Stage #1:glycolic Acid;4-(trifluoromethoxy)benzene-1,2-diamine with hydrogenchloride for 6 h;Reflux;
Stage #2: with ammonium hydroxide in water
Steps:
2-Hydroxymethyl- and 2-ethyl-5-trifluoromethoxybenzimidazole. The procedure of Philips, M. A. was used. A mixture of 2-amino-4-trifluoromethoxyaniline (0.7 g, 0.0036 Mol) and corresponding carboxylic acid (0.00546 Mol) in HCl (3.5 ml, 4N) was refluxed for 6 h. Water (5 mL), and 0.5 g of charcoal were added, and the mixture was refluxed 10-15 min. After cooling, the mixture was filtered and resulted clear filtrate was washed with ether (2×10 mL). The water layer was neutralized with excess of diluted NH4OH, the precipitate was filtered, washed with water, and dried.2-Hydroxymethyl-5-trifluoromethoxybenzimidazole. Glycolic acid was used, R(HO)CH2. Yield 0.65 g (75 wt. %). M.p. 192-194° C. 1H NMR (DMSO-d6): 4.68 (d, 5.5 Hz, 2H), 5.67 (t, 5.5 Hz, 2H), 7.05 (d, J=8.0 Hz, 1H), 7.38 (s, 1H), 7.49 (br s, 1H), 12.48 (br s, 1H). 13C NMR (DMSO-d6): 57.62, 104.44, 110.79, 111.98, 114.70, 118.20, 120.08 (q, J=254.8 Hz), 143.21, 157.22. 19F NMR (DMSO-d6): -57.76. [M+1]+ 233.
References:
The United States of America as represented by the United States Department of Energy US8242284, 2012, B1 Location in patent:Page/Page column 6
2267-23-4
152 suppliers
$7.00/1g

919054-04-9
14 suppliers
inquiry