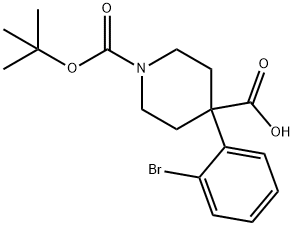
1-BOC-4-(2-BROMOPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID synthesis
- Product Name:1-BOC-4-(2-BROMOPHENYL)-4-PIPERIDINEDICARBOXYLIC ACID
- CAS Number:920023-52-5
- Molecular formula:C17H22BrNO4
- Molecular Weight:384.26

24424-99-5
821 suppliers
$13.50/25G

920023-52-5
21 suppliers
$151.00/250mg
Yield:920023-52-5 92%
Reaction Conditions:
Stage #1: 4-(2-bromo-phenyl)-4-cyano-piperidine-1-carboxylic acid tert-butyl esterwith hydrogenchloride;water for 96 h;Heating / reflux;
Stage #2: di-tert-butyl dicarbonatewith sodium hydroxide in 1,4-dioxane;water at 0 - 20; for 2 h;
Stage #3: with hydrogenchloride in water at 0; pH=1 - 2;
Steps:
282.c
A mixture of 5.7 g (15.6 mmol) 4-(2-bromo-phenyl)-4-cyano-piperidine-1-carboxylic acid tert-butyl ester and 235 ml (936 mmol) of a 4 M aqueous hydrochloric acid solution was heated at reflux for 96 h. After cooling to room temperature the reaction mixture was basified with 93.90 ml (1014 mmol) of a 10.8 M aqueous sodium hydroxide solution and diluted with 200 ml 1,4-dioxane. A solution of 5.11 g (23.4 mmol) di-tert-butyl dicarbonate in 50 ml 1,4-dioxane was added quickly at 0° C. After stirring for 2 h the reaction mixture was extracted with tert-butyl methyl ether (2*100 ml). The combined organic layers were washed with 1 M sodium hydroxide solution (1*100 ml). The combined aqueous layers were cooled by the addition of 100 g ice, acidified (pH 1-2) with ice-cold 2 M aqueous hydrochloric acid solution and extracted with ethyl acetate (3*150 ml). The combined ethyl acetate layers were dried over sodium sulfate and concentrated in vacuo to give the title compound (5.51 g, 92%) as a light yellow solid. MS m/e (%): 382, 384 (M+H+, 90, 100).
References:
US2007/27173,2007,A1 Location in patent:Page/Page column 116