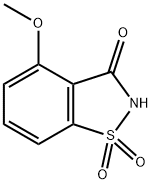
1,2-Benzisothiazol-3(2H)-one,4-methoxy-,1,1-dioxide synthesis
- Product Name:1,2-Benzisothiazol-3(2H)-one,4-methoxy-,1,1-dioxide
- CAS Number:92115-37-2
- Molecular formula:C8H7NO4S
- Molecular Weight:213.21
120257-03-6
2 suppliers
inquiry

124-41-4
703 suppliers
$12.00/25g

92115-37-2
13 suppliers
$60.00/10mg
Yield:92115-37-2 91%
Reaction Conditions:
with methanol at 150; for 0.366667 h;Microwave irradiation;
Steps:
Synthesis of 4-methoxybenzo[d]isothiazol-3(2H)-one1,1-dioxide(8).
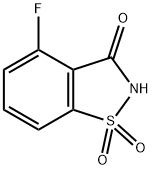
Synthesis of 4-methoxybenzo[d]isothiazol-3(2H)-one1,1-dioxide(8).4-Fluorobenzo[d]isothiazol-3(2H)-one 1,1-dioxide (7) (1 g, 5 mmol) was dissolved in methanol (10 mL) and sodiummethoxide (25% in methanol) (3.41 mL, 14.9 mmol) was added. The clear brown mixture was heated in themicrowave reactor at 150C for 22 min. Themixture was acidified (pH <2) by the addition of hydrochloric acid (1.0 M,aq.) and the solvent was removed. Theresidue was partitioned between hydrochloric acid (1.0M, aq.) and ethyl acetatethen extracted with ethyl acetate. Theorganic layer was dried over magnesium sulfate, filtered and the solventremoved to provide 4-methoxybenzo[d]isothiazol-3(2H)-one 1,1-dioxide (8) (0.968 g, 4.54 mmol, 91 % yield) asa tan solid. LC/MS (Phenomenex Onyx C184.6 x 100 mm column, 10-90% acetonitrile, water with 0.1%TFA gradient over 2min., 5 mL/min.) 0.75 min., m/z 214(M+H); 1H NMR (400MHz, CHLOROFORM-d) d 7.88 - 7.80 (m, 1H), 7.47 (d, J=7.3Hz, 1H), 7.29 (d, J=8.8 Hz, 1H), 4.06 (s, 3H).
References:
Lloyd, John;Finlay, Heather J.;Kover, Alexander;Johnson, James;Pi, Zulan;Jiang, Ji;Neels, James;Cavallaro, Cullen;Wexler, Ruth;Conder, Mary Lee;Shi, Hong;Li, Danshi;Sun, Huabin;Chimalakonda, Anjaneya;Huang, Christine;Salvati, Mark;Levesque, Paul [Bioorganic and Medicinal Chemistry Letters,2015,vol. 25,# 21,p. 4983 - 4986] Location in patent:supporting information

133742-77-5
0 suppliers
inquiry

92115-37-2
13 suppliers
$60.00/10mg

51674-10-3
14 suppliers
$53.20/250mg

92115-37-2
13 suppliers
$60.00/10mg

701-27-9
230 suppliers
$6.00/1g

92115-37-2
13 suppliers
$60.00/10mg