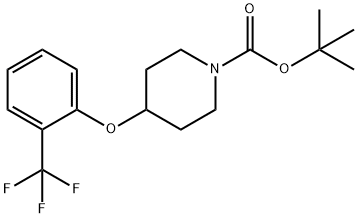
tert-butyl 4-(2-(trifluoromethyl)phenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-butyl 4-(2-(trifluoromethyl)phenoxy)piperidine-1-carboxylate
- CAS Number:921605-76-7
- Molecular formula:C17H22F3NO3
- Molecular Weight:345.36
Yield:921605-76-7 64%
Reaction Conditions:
with triphenylphosphine;diethylazodicarboxylate in tetrahydrofuran at 20 - 25; for 16 h;
Steps:
46.1 Step 1: Preparation of tert-butyl 4-(2-(trifluoromethyl)phenoxy)piperidine-1-carboxylate
To a solution of 2-(trifluoromethyl)phenol (1.61 g, 9.94 mmol) in tetrahydrofuran (40 mL) at 25 C was added tert-butyl 4-hydroxypiperidine-1-carboxylate (2.0 g, 9.94 mmol), diethyl azodicarboxylate (2.60 g, 14.9 mmol) and triphenylphosphine (3.91 g, 14.9 mmol). The reaction mixture was stirred at room temperature for 16 h, then diluted with water (200 mL). The aqueous layer was extracted with dichloromethane (80 mL × 3). The combined organic layers were washed with brine (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated in vacuo. The crude material was purified by column chromatography (silica gel, petroleum ether / ethyl acetate = 1/1) to afford tert-butyl 4-(2- (trifluoromethyl)phenoxy)piperidine-1-carboxylate (2.2 g, 6.37 mmol, 64%) as brown solid. LCMS (ESI) m/z: 346.2 [M+H]+.
References:
WO2020/154571,2020,A1 Location in patent:Page/Page column 115; 116

