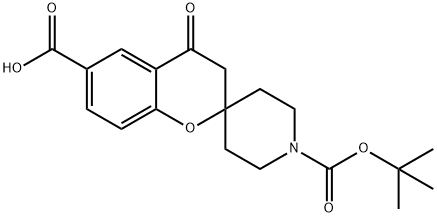
3,4-DIHYDRO-4-OXO-SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDINE]-1',6-DICARBOXYLIC ACID 1'-(1,1-DIMETHYLETHYL) ESTER synthesis
- Product Name:3,4-DIHYDRO-4-OXO-SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDINE]-1',6-DICARBOXYLIC ACID 1'-(1,1-DIMETHYLETHYL) ESTER
- CAS Number:921760-85-2
- Molecular formula:C19H23NO6
- Molecular Weight:361.39
![Spiro[2H-1-benzopyran-2,4'-piperidine]-6-carboxylic acid, 3,4-dihydro-4-oxo-, hydrochloride (1:1)](/CAS/20211123/GIF/921760-84-1.gif)
921760-84-1
0 suppliers
inquiry

24424-99-5
823 suppliers
$13.50/25G
![3,4-DIHYDRO-4-OXO-SPIRO[2H-1-BENZOPYRAN-2,4'-PIPERIDINE]-1',6-DICARBOXYLIC ACID 1'-(1,1-DIMETHYLETHYL) ESTER](/CAS/GIF/921760-85-2.gif)
921760-85-2
17 suppliers
$183.00/0.25/ G
Yield:-
Reaction Conditions:
Stage #1: 4-oxospiro[chroman-2,4'-piperidine]-6-carboxylic acid hydrochloride;di-tert-butyl dicarbonatewith sodium hydrogencarbonate in 1,4-dioxane;water at 20; for 13 h;
Stage #2: with hydrogenchloride;water
Steps:
46
Reference Example 46: tert-butyl 6-carboxy-4-oxospiro(chroman-2.4'-ρiperidine)- 1 ' carboxylate.; 6- Carboxy-4-oxospiro(chroman-2,4'-piperidine) hydrochloride (15.0 g, 50.3 mmol) was dissolved in 1,4- dioxane (150 ml) and H2O (150 ml), NaHCO3 (10.6 g, 126 mmol) and DlBOC (13.2 g, 60.4 mmol) were added threreto in this order. After stirred at room temperature for 13 h, the reaction mixture was diluted with Et2θ and 5N NaOH aq. (12.1 ml). The aqueous layer was washed with Et2θ and acidified with 6N HCl aq. (PH=ca. 3), then extracted with CHCI3. The organic layer was washed with brine and dried over EPO
References:
WO2007/11809,2007,A1 Location in patent:Page/Page column 101-102