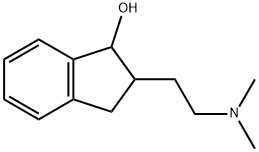
1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro- synthesis
- Product Name:1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-
- CAS Number:92196-07-1
- Molecular formula:C13H19NO
- Molecular Weight:205.3
867038-07-1
0 suppliers
inquiry
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry
Yield:-
Reaction Conditions:
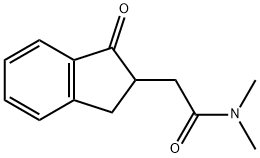
Stage #1: N,N-dimethyl-2-(1-oxo-indan-2-yl)-acetamidewith lithium aluminium hydride in tetrahydrofuran at 0 - 20; for 6.5 h;Heating / reflux;
Stage #2: with sodium hydroxide;lithium hydroxide monohydrate at 20; for 0.5 h;
Steps:
3
To a solution of LiAlH4 (7.8 g, 200 mmol) in THF (300 mL) at 0 °C, N, N- dimethyl-2- (1-oxo-indan-2-yl)-acetamide la (10 g, 46 mmol) in THF (40 mL) was added slowly. The reaction was allowed to stir at room temperature for half an hour and then at reflux for 6 hours. The reaction was cooled to room temperature and carefully quenched by the sequential addition of H20 (7.6 mL), 15% NaOH (7.6 mL) and H20 (22.8 mL). The mixture was stirred for a half hour, and the solid was filtered off using a celite pad and washed with excess THF. The combined filtrates were dried overNa2S04 and concentrated to provide 2-(2-dimethylamino-ethyl)-indan- I -ol 2a as a yellow oil. 2-(2-Dimethylamino- ethyl)-indan- I -ol 2a was dissolved in acetic acid (120 mL) and concentrated HCl (40 mL), and the resultant mixture was refluxed for 2 hours. Most of the solvent was evaporated in vacuo. The residue was diluted with water (200 mL) and washed with ether (50 mL). The aqueous solution was basified with NH40H, extracted twice with ether (250 mL), dried over Na2S04, concentrated and purified by flash chromatography (CHCl3:MeOH:NH4OH, 98: 2:0.2) to give a pale yellow oil, [2-(1H-inden-2-yl)-ethyl]-dimethyl-amine 3a, in 83 % yield. (MH+ =188.0) In a similar manner [2-(6-methoxy-lH-inden-2-yl)-ethyl]-dimethyl-amine 3b was prepared in 15% yield. MH+ = 217.9
References:
WO2005/97093,2005,A1 Location in patent:Page/Page column 33-34
83-33-0
549 suppliers
$6.00/5g
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry
![2-[2-(DiMethylaMino)ethyl]-1-indanone](/CAS/GIF/3409-21-0.gif)
3409-21-0
36 suppliers
$139.80/10mg
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry
-, diethyl ester (9CI)](/CAS/20200515/GIF/1805-03-4.gif)
1805-03-4
1 suppliers
inquiry
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry

607-81-8
222 suppliers
$9.00/1g
![1H-Inden-1-ol, 2-[2-(dimethylamino)ethyl]-2,3-dihydro-](/CAS/20210111/GIF/92196-07-1.gif)
92196-07-1
0 suppliers
inquiry