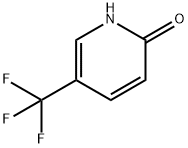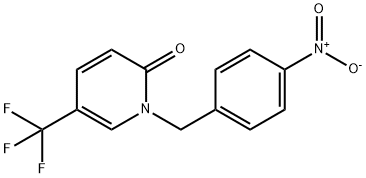
2(1H)-Pyridinone, 1-[(4-nitrophenyl)methyl]-5-(trifluoromethyl)- synthesis
- Product Name:2(1H)-Pyridinone, 1-[(4-nitrophenyl)methyl]-5-(trifluoromethyl)-
- CAS Number:923688-20-4
- Molecular formula:C13H9F3N2O3
- Molecular Weight:298.22
Yield:923688-20-4 96.9%
Reaction Conditions:
with potassium carbonate in dimethyl sulfoxide at 130; for 4 h;
Steps:
1.1. General procedure for the synthesis of compound (1), (2) and (18).
General procedure: A mixture of 5-trifluoromethyl-2 (1H)-pyridone(20 mmol)and potassium carbonate (32 mmol) in DMSO (100 mL) reacted with 3-chloro-4-fluoro-nitrobenzene, 1-(chloromethyl)-4-nitrobenzene or1-(chloromethyl)-2-nitrobenzene (30 mmol) at 130.After cooling the reaction solution was diluted with 12% ammonia300 mL. The precipitate was filtrated off . The residue was recrystallized with EtOAc to give compounds(1, 2, 18) respectively.
References:
Chen, Jun;Peng, Zhangzhe;Lu, Miaomiao;Xiong, Xuan;Chen, Zhuo;Li, Qianbin;Cheng, Zeneng;Jiang, Dejian;Tao, Lijian;Hu, Gaoyun [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 2,p. 222 - 229] Location in patent:supporting information
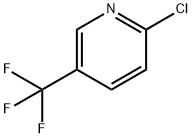
52334-81-3
426 suppliers
$5.00/1g
![2(1H)-Pyridinone, 1-[(4-nitrophenyl)methyl]-5-(trifluoromethyl)-](/CAS/20200611/GIF/923688-20-4.gif)
923688-20-4
1 suppliers
inquiry