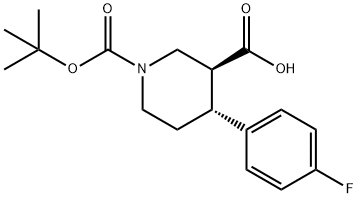
(3S,4R)-1-(tert-Butoxycarbonyl)-4-(4-fluorophenyl)-piperidine-3-carboxylic acid synthesis
- Product Name:(3S,4R)-1-(tert-Butoxycarbonyl)-4-(4-fluorophenyl)-piperidine-3-carboxylic acid
- CAS Number:923932-21-2
- Molecular formula:C17H22FNO4
- Molecular Weight:323.36
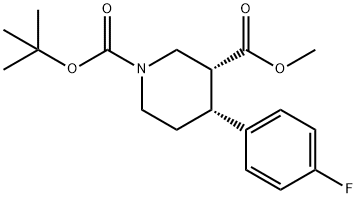
951167-04-7
1 suppliers
inquiry

923932-21-2
10 suppliers
inquiry
Yield:923932-21-2 52%
Reaction Conditions:
Stage #1: (+)-(3R,4R)-4-(4-fluoro-phenyl)-piperidine-1,3-dicarboxylic acid 1-tert butyl ester 3-methyl esterwith sodium methylate in toluene;Heating / reflux;
Stage #2: with sodium hydroxide;water in 1,4-dioxane at 20; for 5 h;
Stage #3: with hydrogenchloride in water at 0; pH=1 - 2;Product distribution / selectivity;
Steps:
1.c
c) (-)-(3S,4R)-4-(4-Fluoro-phenyl)-piperidine-1,3-dicarboxylic acid 1-tert-butyl ester A mixture of (+)-(3R,4R)-4-(4-fluoro-phenyl)-piperidine-1,3-dicarboxylic acid 1-tert butyl ester 3-methyl ester (3.55 g, 10.5 mmol) and sodium methoxide (1.14 g, 21.1 mmol) in 100 ml anhydrous toluene was heated at reflux over night. After cooling to room temperature the reaction mixture was quenched with water and concentrated in vacuo. The residue was dissolved in a mixture of 100 ml 1,4-dioxane and 50 ml 2 M aqueous sodium hydroxide solution. After stirring at RT for 5 h the mixture was diluted with water and washed with two portions of tert-butyl methyl ether. The aqueous layer was cooled to 0° C., acidified to pH 1-2 with ice-cold 1 M aqueous hydrochloric acid solution and extracted with three portions of tert-butyl methyl ether. The combined organic layers were dried over sodium sulfate and concentrated in vacuo. Flash column chromatography and crystallization from heptane/ethyl acetate 9:1 (30 ml) gave the title compound as white crystals (1.76 g, 52%, 97.5% ee). MS m/e (%): 322 (M-H+, 100).
References:
US2007/232652,2007,A1 Location in patent:Page/Page column 14
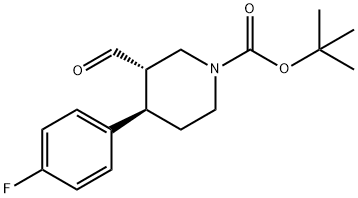
923932-19-8
0 suppliers
inquiry

923932-21-2
10 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G

923932-21-2
10 suppliers
inquiry
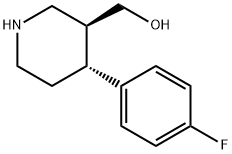
125224-43-3
89 suppliers
$49.00/25mg

923932-21-2
10 suppliers
inquiry
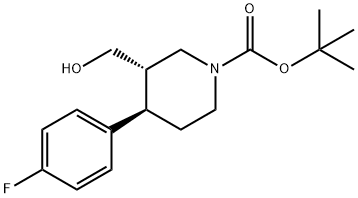
200572-33-4
39 suppliers
$226.00/250mg

923932-21-2
10 suppliers
inquiry