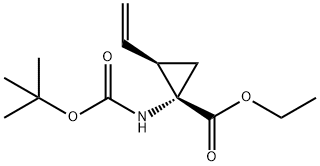
ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate synthesis
- Product Name:ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate
- CAS Number:924307-75-5
- Molecular formula:C13H21NO4
- Molecular Weight:255.31
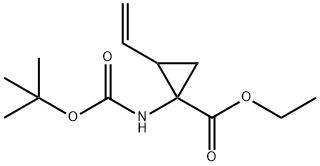
681807-59-0
71 suppliers
inquiry
![Cyclopropanecarboxylic acid, 1-[[(1,1-dimethylethoxy)carbonyl]amino]-2-ethenyl-, ethyl ester, (1R,2S)-](/CAS/GIF/259217-95-3.gif)
259217-95-3
79 suppliers
$13.00/250mg
![ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate](/CAS/20180527/GIF/924307-75-5.gif)
924307-75-5
6 suppliers
inquiry
Yield:259217-95-3 49.9% ,924307-75-5 25.9%
Reaction Conditions:
with ammonium hydroxide;carbon dioxideSupercritical conditions;
Steps:
22.4 Synthesis of compounds 37D and 37E
The compound 37C (1 g) was resolved by SFC to give two isomers. SFC method: Column: AY(250mm *30mm, 1 Oum) Mobile phase: A: CO7 13:0.1 %NH3H2O ETOH; Gradient: 15% of B; Flow rate: 6OmL/inin.The compound 37C was separated by SFC to give compound 371) (500 mg, yield:49.9%) (Rt 149 mm) and compound 37E (450 mg, yield: 259%) (Rt == 2.68 mm) both asyellow oil. ‘1-1 NMR (400MHz, DMSO-d6) 764 (s, IH), 567- 554 (m, 1FF). 5.21 (hr d, J::::17.2 Hz, 1H), 5.07-5.01 (m, 1H), 4.10- 394 (rn, 2H), 2.08 (q, J:::: 8.7 Hz, lET), 157- 1.44(m, 1H), 1.38- 1.31 (m, 9H), 127- 123 (m, 1H), 1.18- 1.10 (m, 3H). MS (ESI m/z (M100Y 155.8.‘HMR (400’llTz, DMSO-d6) 5 7.64 (s, 1H), 5.67- 5.54 (m, IH), 5.21 (hr d, J=17.0 Hz, 1H), 5.04 (dd,J= 1.7, 10.3 Hz, 1H), 4.11 -3.95 (m, 2H), 2.08 (q,J= 8.8 Hz, IH),1.54 (brdd,J= 5.4, 7.2 Hz, IH), 1.38- 1.31 (m, 9H), 1.27 -1.23 (m, IH), 1.19- 1.10 (m,3H). MS (ESI) m/ (M-i00 1559..
References:
WO2017/156074,2017,A1 Location in patent:Paragraph 00521

681807-59-0
71 suppliers
inquiry
![ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate](/CAS/20180527/GIF/924307-75-5.gif)
924307-75-5
6 suppliers
inquiry
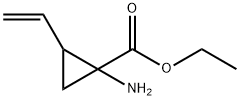
787548-29-2
37 suppliers
inquiry

24424-99-5
832 suppliers
$13.50/25G
![Cyclopropanecarboxylic acid, 1-[[(1,1-dimethylethoxy)carbonyl]amino]-2-ethenyl-, ethyl ester, (1R,2S)-](/CAS/GIF/259217-95-3.gif)
259217-95-3
79 suppliers
$13.00/250mg
![ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate](/CAS/20180527/GIF/924307-75-5.gif)
924307-75-5
6 suppliers
inquiry
![Cyclopropanecarboxylic acid, 1-[(diphenylmethylene)amino]-2-ethenyl-, ethyl ester](/CAS/20210305/GIF/1023795-84-7.gif)
1023795-84-7
0 suppliers
inquiry
![ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate](/CAS/20180527/GIF/924307-75-5.gif)
924307-75-5
6 suppliers
inquiry
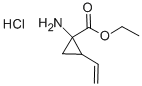
681807-60-3
15 suppliers
inquiry
![ethyl (1S,2R)-1-{[(tert-butoxy)carbonyl]amino}-2-ethenylcyclopropane-1-carboxylate](/CAS/20180527/GIF/924307-75-5.gif)
924307-75-5
6 suppliers
inquiry