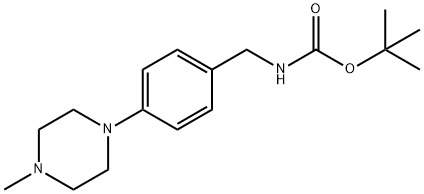
tert-Butyl 4-(4-Methylpiperazin-1-yl)benzylcarbaMate synthesis
- Product Name:tert-Butyl 4-(4-Methylpiperazin-1-yl)benzylcarbaMate
- CAS Number:927676-52-6
- Molecular formula:C17H27N3O2
- Molecular Weight:305.42
216144-45-5
104 suppliers
$14.14/250mgs:

24424-99-5
823 suppliers
$13.50/25G

927676-52-6
23 suppliers
$24.00/5g
Yield:927676-52-6 82%
Reaction Conditions:
with sodium hydroxide in tetrahydrofuran;water at 20; for 1 h;
Steps:
F.F (1)
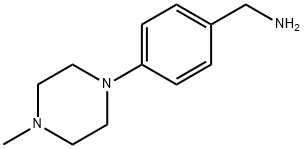
Preparation F; (3-chloro-4-(4-methylpiperazin-1-yl)phenyl)methanamine; Step F (1); To a solution of (4-(4-methylpiperazin-1-yl)phenyl)methanamine (204 mg, 1.0 mmol, Aldrich) in THF (2.0 mL) was added (Boc)2O (327 mg, 1.5 mmol) and NaOH/H2O (1.0 mL, 1 N). The mixture was stirred at rt for 1 h. EtOAc (100 mL) was added and the resulting solution was washed with H2O (2×100 mL). The organic layer was dried and concentrated to give 250 mg (yield 82%) of tert-butyl 4-(4-methylpiperazin-1-yl)benzylcarbamate. LC-MS (M+H)+=306.35 1H-NMR(500 MHz, CD3OD) δ 7.18 (d, J=8.55 Hz, 2H), 6.94 (d, J=8.54 Hz, 2H), 4.15 (s, 2H), 3.16-3.22 (m, 4H), 2.60-2.66 (m, 4H), 2.36 (s, 3H), 1.46 (s, 9H).
References:
US2007/49589,2007,A1 Location in patent:Page/Page column 13