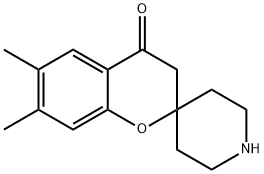
6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine] synthesis
- Product Name:6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine]
- CAS Number:927978-38-9
- Molecular formula:C15H19NO2
- Molecular Weight:245.32
![tert-butyl 6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/1013333-61-3.gif)
1013333-61-3
7 suppliers
inquiry
![6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine]](/CAS/20150408/GIF/927978-38-9.gif)
927978-38-9
9 suppliers
inquiry
Yield:927978-38-9 78.6%
Reaction Conditions:
Stage #1: tert-butyl 6,7-dimethyl-4-oxo-3,4-dihydro-1'H-spiro[chromene-2,4'-piperidine]-1'-carboxylatewith trifluoroacetic acid in dichloromethane at 20;
Stage #2: with sodium hydroxide in water; pH=~ 14;
Steps:
22
1-(2-Hydroxy-4,5-dimethylphenyl)ethanone (150 g, 0.914 mol) and pyrrolidine (76.3 mL, 0.914 mol) in methanol (1 L) were stirred for 15 minutes. Then tert-butyl 4-oxopiperidine-1-carboxylate (182.2 g, 0.914 mol) was added and the mixture was stirred for 24 hours. The formed precipitate was separated by filtration, washed with hexane and dried to give 245 g of pure tert-butyl 6,7-dimethyl-4-oxo-3,4-dihydro- 1'H-spirofchromene-2,4'-piperidine]-1'-carboxylate. The combined filtrates were evaporated, and the residue was crystallized from some hexane to give an additional portion (21 g) of product. The total yield was 77.1 % (266 g). 1H NMR (CDCI3) δ 7.58 (s, 1 H), 6.76 (s, 1 H), 3.84 (br d, J=13, 2H), 3.18 (m, 2 H), _2J64_(S,.2H),_Z25_(S,JH), 2J.9Js,AH),l,99Jbrji,JrJ2,_2H), 1.59.(m, 2H),.1.44 (s, 9H), 344 (API-).Neat trifluoroacetic acid (80 mL) was added under cooling with cold water to tert-butyl 6,7-dimethyl-4- oxo-3,4-dihydro-1η-spiro[chromene-2,4'-piperidine]-1'-carboxylate (50 g, 0.145 mol) in dichloromethane (300 mL). The mixture was stirred at room temperature overnight and then the volatiles were evaporated. The residue was dissolved in water and the aqueous layer washed twice with ether, then made alkaline ~Witl"fNaOH to about pH 14. The product was extracted with" CHCI3, dried (Na2SO4), and concentrated to afford the product (27.9 g; 78.6%). 1H NMR (DMSO-d6) δ 7.49 (s, 1H), 6.94 (s, 1H), 3.34 (br s, 6 H), 3.95 (br d, J=12, 1H), 3.82 (t, J=12, 1 H), 2.82 (s, 1H), 2.24 (s, 2H), 2.18 (s, 2H), 2.59 (d, J=15, 1H), 2.34 (t, J=11 , 1 H). LC/MS API+ 246 (MH+).
References:
WO2008/65508,2008,A1 Location in patent:Page/Page column 22
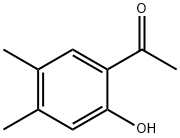
36436-65-4
151 suppliers
$29.00/500mg
![6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine]](/CAS/20150408/GIF/927978-38-9.gif)
927978-38-9
9 suppliers
inquiry

79099-07-3
543 suppliers
$5.00/5g
![6,7-diMethyl-4- oxo-3,4-dihydro-1η-spiro[chroMene-2,4'-piperidine]](/CAS/20150408/GIF/927978-38-9.gif)
927978-38-9
9 suppliers
inquiry