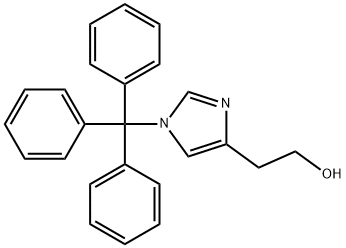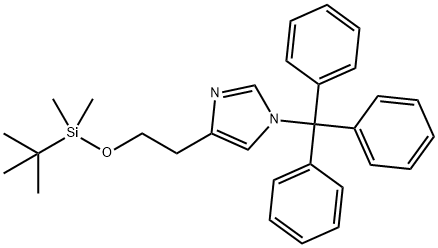
4-(2-(tert-butyldiMethylsilyloxy)ethyl)-1-trityl-1H-iMidazole synthesis
- Product Name:4-(2-(tert-butyldiMethylsilyloxy)ethyl)-1-trityl-1H-iMidazole
- CAS Number:928136-82-7
- Molecular formula:C30H36N2OSi
- Molecular Weight:468.71
Yield:928136-82-7 77%
Reaction Conditions:
with 1H-imidazole in dichloromethane at 25;
Steps:
1.3 EXAMPLE 1
[00147j 4-(2-(tert-butvldimethylsilyloxy)ethyl)- 1 -trityl- 1H-imidazole: 2-(1 -Trityl-1H- imidazol-4-yl) ethanol (35 g, 98.75 mmol, 1.00 equiv) was dissolved in DCM (210 mL). To this was added imidazole (19.95 g, 293.05 mmol, 3.00 equiv) and tertbutyldimethylsilylchloride (22.40 g, 149.27 mmol, 1.50 equiv). The mixture was stirred at room temperature until LCMS indicated completion of the reaction. Then the resulting solution was diluted with 500 mL of DCM. The resulting mixture was washed with water (3 x 300 mL). The residue was purified by a silica gel column, eluted with ethyl acetate/petroleum ether (1:4) to afford 40 g (77%) of 4-[2-[(tert- butyldimethylsilyl)oxyj ethyll -1 -(triphenylmethyl)- 1H-imidazole as a white solid. LCMS (ESI): m/z = 469.1 [M+Hf
References:
WO2016/109361,2016,A2 Location in patent:Paragraph 00147

76-83-5
687 suppliers
$6.00/25g

928136-82-7
9 suppliers
inquiry

168632-03-9
13 suppliers
inquiry

928136-82-7
9 suppliers
inquiry
3251-69-2
206 suppliers
$15.00/250mg

928136-82-7
9 suppliers
inquiry