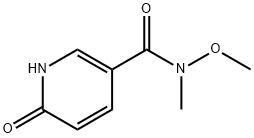
N-methoxy-N-methyl-6-oxo-1,6-dihydropyridine-3-carboxamide synthesis
- Product Name:N-methoxy-N-methyl-6-oxo-1,6-dihydropyridine-3-carboxamide
- CAS Number:928794-16-5
- Molecular formula:C8H10N2O3
- Molecular Weight:182.18
Yield:928794-16-5 67%
Reaction Conditions:
with pyridine in chloroform at 0 - 20; for 16 h;
Steps:
9
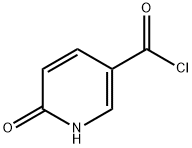
6-Hvdroxy-N-methoxy-N-methyl-nicotinamide; Add 6-hydroxy-nicotinoyl chloride (11.2 g, 71 mmol) and N,O-dimethylhydroxylamine hydrochloride (7.76 g, 79.5 mmol) to ethanol-free chloroform (100 mL, wash chloroform with water, dry over MgSO4, then filter through neutral alumina to obtain ethanol-free chloroform). Cool the mixture to 0°C under a nitrogen atmosphere. Add pyridine (12.6 mL, 155.8 mmol), warm reaction up to room temperature and stir for 16 h under a nitrogen atmosphere. Wash reaction with water (2x75 mL) followed by brine (75 mL). Dry the organic layer over MgSO4, filter and concentrate in vacuo. Purify the crude mixture by chromatography on silica gel EPO
References:
WO2007/28082,2007,A1 Location in patent:Page/Page column 65-66
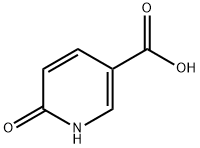
5006-66-6
465 suppliers
$13.43/1gm:

6638-79-5
560 suppliers
$6.00/25g

928794-16-5
1 suppliers
inquiry