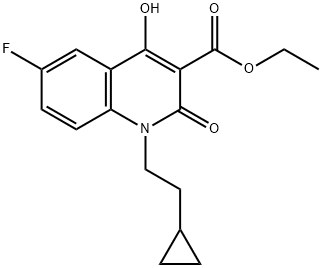
Ethyl 1-(2-Cyclopropylethyl)-6-fluoro-4-hydroxy-2-oxo-1,2-dihydro-3-quinolinecarboxylate synthesis
- Product Name:Ethyl 1-(2-Cyclopropylethyl)-6-fluoro-4-hydroxy-2-oxo-1,2-dihydro-3-quinolinecarboxylate
- CAS Number:931399-20-1
- Molecular formula:C17H18FNO4
- Molecular Weight:319.33
![1-(2-CYCLOPROPYL-ETHYL)-6-FLUORO-1H-BENZO[D][1,3]OXAZINE-2,4-DIONE](/CAS/GIF/477933-12-3.gif)
477933-12-3
22 suppliers
$150.00/50mg
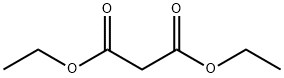
105-53-3
741 suppliers
$5.00/25g

931399-20-1
6 suppliers
$150.00/10mg
Yield:931399-20-1 83%
Reaction Conditions:
Stage #1: diethyl malonatewith sodium hydride in N,N-dimethyl acetamide at 10 - 20; for 0.5 h;
Stage #2: 1-(2-cyclopropylethyl)-6-fluoro-1H-benzo[d][1,3]oxazine-2,4-dione in N,N-dimethyl acetamide at 120; for 20 h;
Steps:
34.34a
A suspension of hexanes-washed sodium hydride (20.1 g, 60% dispersion in mineral oil, 0.50 mole) in dry N,N-dimethylacetamide (250 mL) was cooled to 10 0C then treated drop wise with a solution of diethyl malonate (80.3 g, 0.50 mole) in N,N-dimethylacetamide (300 mL) at such a rate to maintain the temperature < 20 0C. The solution was stirred at ambient temperature for 30 min. then treated dropwise with a solution of the l-(2-cyclopropylethyl)-6-fluoro-2H-3,l-benzoxazine-2,4(lH)- dione (WO02/098424) (100 g, 0.40 mol.) in dry N,N-dimethylacetamide (500 mL) and the mixture was stirred and heated at 120 0C for 20 h. The mixture was allowed to cooled to 60 0C, then acidified with glacial acetic acid (40.0 mL), and poured into water (2.5 L). The mixture was left to stand for Ih, then filtered and dried in vacuo. The solid was then washed with hexanes (1 L) and dried in vacuo to afford the title compound as a cream solid (105.4 g, 83%). 1H NMR (400 MHz, DMSOd6) δ ppm 12.75 (s, 1 H), 7.74 - 7.80 (m, 1 H), 7.62 (s, 1 H), 7.61 (dd, J=4.9, 2.4 Hz, 1 H), 4.30 (q, J=7.1 Hz, 2 EPO
References:
WO2007/38571,2007,A2 Location in patent:Page/Page column 38-39