
Tert-butyl (2-((4-(trifluoroMethoxy)benzyl)aMino)ethyl)carbaMate synthesis
- Product Name:Tert-butyl (2-((4-(trifluoroMethoxy)benzyl)aMino)ethyl)carbaMate
- CAS Number:934757-43-4
- Molecular formula:C15H21F3N2O3
- Molecular Weight:334.33
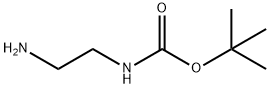
57260-73-8
465 suppliers
$5.00/5g
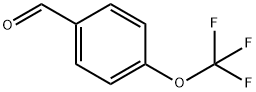
659-28-9
274 suppliers
$15.00/5g

934757-43-4
17 suppliers
$154.53/250mg
Yield:934757-43-4 88%
Reaction Conditions:
Stage #1: N-BOC-1,2-diaminoethane;p-trifluoromethoxybenzaldehyde in dichloromethane; for 1 h;
Stage #2: with sodium tris(acetoxy)borohydride in dichloromethane at 20; for 4 h;
Stage #3: with sodium hydroxide;water in ethyl acetate;
Steps:
3.1
4-{2-[Pentyl-(4-trifluoromethoxy-benzyl)-amino]-ethylsulfamoyl}-indan-2-carboxylic acidStep 1 [2-(4-Trifluoromethoxy-benzylamino)-ethyl]-carbamic acid tert-butyl ester: To a solution of 4-(trifluoromethoxy)-benzaldehyde (237 mg, 1.25 mmol) in methylene chloride (30 mL) was added N-(2-aminoethyl)carbamic acid tert-butyl ester (200 mg, 1.25 mmol). After 1 h sodium triacetoxy borohydride (527 mg, 2.50 mmol) was added and the reaction mixture was stirred at room temperature for 4 h. The reaction mixture was concentrated, diluted with ethyl acetate, washed with 1N NaOH and concentrated in vacuo. The residue was purified by silica gel chromatography (0-50% ethyl acetate in hexanes) to afford [2-(4-trifluoromethoxy-benzylamino)-ethyl]-carbamic acid tert-butyl ester (213 mg, 88%) as a clear oil. 1H NMR (400 MHz, CDCl3) δ 7.34 (d, 2H), 7.16 (d, 2H), 4.90 (m, 2H), 3.80 (s, 2H), 3.28-3.10 (m, 2H), 2.86-2.70 (m, 2H), 1.45 (s, 9H); LCMS 335.5 (M+1)+.
References:
US2008/287477,2008,A1 Location in patent:Page/Page column 14