4-(4-ethoxycarbonylphenyl)azobenzoic acid ethyl ester synthesis
- Product Name:4-(4-ethoxycarbonylphenyl)azobenzoic acid ethyl ester
- CAS Number:936-47-0
- Molecular formula:C9H10N2
- Molecular Weight:146.19
104-55-2
652 suppliers
$6.00/25g

936-47-0
9 suppliers
inquiry
Yield:936-47-0 85%
Reaction Conditions:
with hydrazine in tert-butyl alcohol at 80; for 6 h;
Steps:
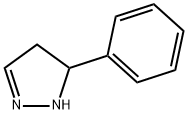
1 5-phenyl-4,5-dihydro-lH-pyrazole (I-E):
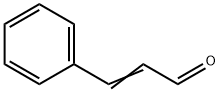
Hydrazine (0.684 mL, 19 mmol) was heated to reflux. A solution of cinnamaldehyde (1.0 g, 7.6 mmol) in tert-butanol (20 mL) was added dropwise, and the mixture was refluxed for 6 hours. The reaction mixture was concentrated under reduced pressure. The crude material was then diluted with DCM (2x100 mL) and washed with water. The combined organic layers were washed with water and then dried over Na2SO4and concentrated under reduced pressure to provide the title compound (0.94 g, 85% yield) of a yellow oil. The product was carried onto the next reaction without furflier purification. MS (ESI) m/z; [M + H]+, 147.2
References:
WO2022/120118,2022,A1 Location in patent:Page/Page column 119; 121