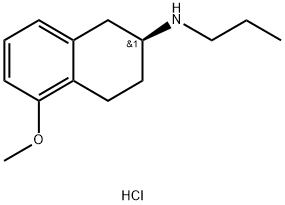
(S)-1,2,3,4-Tetrahydro-5-methoxy-N-propyl-2-naphthalenamine hydrochloride synthesis
- Product Name:(S)-1,2,3,4-Tetrahydro-5-methoxy-N-propyl-2-naphthalenamine hydrochloride
- CAS Number:93601-86-6
- Molecular formula:C14H22ClNO
- Molecular Weight:255.79
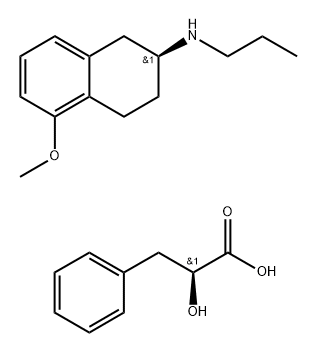
1352917-67-9
1 suppliers
inquiry

93601-86-6
89 suppliers
$140.00/50mg
Yield: 84%
Reaction Conditions:
Stage #1:(S)-1,2,3,4-tetrahydro-5-methoxy-N-propyl-naphthalen-2-ammonium (S)-2-hydroxy-3-phenylpropionate with sodium hydroxide in 2-methyltetrahydrofuran;water at 20;
Stage #2: with hydrogenchloride in 2-methyltetrahydrofuran;water at 60;
Steps:
5.a References:
UCB PHARMA GMBH;ATES, Célal;SCHULE, Arnaud;PALACIO, Magali;DEUTSCH, Paul;VASSELIN, David;CARLY, Nicolas;PHADTARE, Ganesh;YERANDE, Swapnil;DELATINNE, Jean-Pierre;ESCUDERO HERNANDEZ, Maria Luisa;PINILLA, Véronique WO2011/161255, 2011, A2 Location in patent:Page/Page column 31
Example 5a. Preparation of substantially optically pure ((S)-1 ,2,3,4-tetrahydro-5- methoxy-N-propyl- naphthalen-2-ammonium hydrochloride (la) from salt (Vac); The isolated diastereomeric salt (S)-1,2,3,4-tetrahydro-5-methoxy-N-propyl- naphthalen-2-ammonium (S)-2-hydroxy-3-phenylpropionate (Vac) was suspended into a mixture of Me-THF (5 vol) and water (2 vol). Sodium hydroxide (1.1 eq) was added as a solid. The resulting suspension was stirred at room temperature until complete solubilisation. The layers were let to settle and the aqueous layer was discarded. The organic layer was washed twice with deionised water (2 vol), then heated at 60°C. (la) was formed by addition of a 37% solution of hydrochloric acid (1.1 eq.). After cooling at 0-10°C, the resulting solid was filtered, washed twice with Me-THF and dried under vacuum to afford (S)-1,2,3,4-tetrahydro-5-methoxy-N-propyl- naphthalen-2-amine hydrochloride (la) in (yield : 84%.; chiral HPLC : 100 % of (la)).NMR 1H (dmso

1352917-66-8
0 suppliers
inquiry

93601-86-6
89 suppliers
$140.00/50mg
1352917-68-0
1 suppliers
inquiry

93601-86-6
89 suppliers
$140.00/50mg
101403-24-1
40 suppliers
inquiry

93601-86-6
89 suppliers
$140.00/50mg
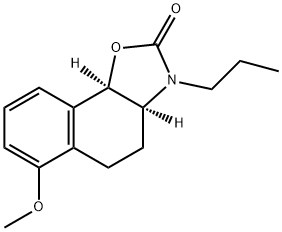
1256261-00-3
1 suppliers
inquiry

93601-86-6
89 suppliers
$140.00/50mg