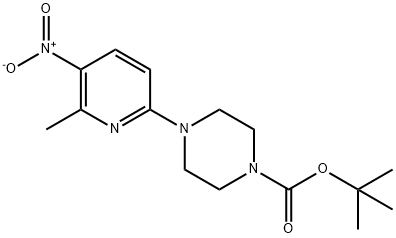
4-(6-METHYL-5-NITRO-2-PYRIDINYL)-1-PIPERAZINECARBOXYLIC ACID 1,1-DIMETHYLETHYL ESTER synthesis
- Product Name:4-(6-METHYL-5-NITRO-2-PYRIDINYL)-1-PIPERAZINECARBOXYLIC ACID 1,1-DIMETHYLETHYL ESTER
- CAS Number:936368-55-7
- Molecular formula:C15H22N4O4
- Molecular Weight:322.36
22280-60-0
319 suppliers
$10.00/1g

57260-71-6
740 suppliers
$5.00/5g

936368-55-7
5 suppliers
inquiry
Yield:936368-55-7 99%
Reaction Conditions:
with triethylamine in butan-1-ol at 20 - 65;
Steps:
3
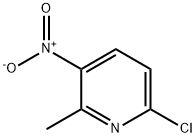
Mechanically stir mixture of 6-chloro-2-methyl-3-nitro-pyridine (Asymchem, 25.00 g, 144.9 mmol), tert-butyl 1-piperazinecarboxylate (29.68 g, 159.4 mmol), and triethylamine (23.2 niL, 167 mmol) in K-BuOH (250 mL) at 50 °C under nitrogen for 4 hours, at 65 °C for 2 hours, then at room temperature overnight. Add additional tert-butyl 1-piperazinecarboxylate (1.5 g), and heat the reaction at 65 °C for 4 hours. Cool the resulting slurry to 30 °C then add hexanes (75 mL). Cool the slurry to room temperature over 1 hour, then add water (150 mL). Allow the slurry to stand for 45 min then filter. Wash the cake with water (4 x 100 mL) then hexanes (100 mL) and air-dry overnight to yield 4-(6-methyl-5-nitro-pyridin-2-yl)- ρiperazine-1-carboxylic acid tert-butyl ester as bright yellow crystals (46.30 g, 99% yield, MS(ES): m/z = 267 [MH-H-C4H8]).
References:
WO2007/53394,2007,A1 Location in patent:Page/Page column 13