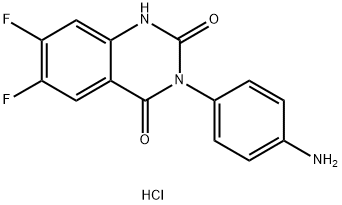
3-(4-AMinophenyl)-6,7-difluoroquinazoline- 2,4(1H,3H)-dione hydrochloride synthesis
- Product Name:3-(4-AMinophenyl)-6,7-difluoroquinazoline- 2,4(1H,3H)-dione hydrochloride
- CAS Number:936500-99-1
- Molecular formula:C14H10ClF2N3O2
- Molecular Weight:325.7
![Carbamic acid, N-[4-(6,7-difluoro-1,4-dihydro-2,4-dioxo-3(2H)-quinazolinyl)phenyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/936500-98-0.gif)
936500-98-0
0 suppliers
inquiry

936500-99-1
22 suppliers
inquiry
Yield:936500-99-1 96%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane at 20 - 30; for 5 - 6 h;Product distribution / selectivity;
Steps:
2.3; 3.2
The N-Boc-aniline 4a (4.Og, 10.28 mmol) was placed in a round-bottomed, flask and 4N HCl in dioxane (50.0 mL, 200 mmol, 19.40 equiv) was added. The heavy, negligibly solvated suspension was stirred at room temperature for 5.0 h. HPLC showed no starting material and clean formation of the aniline 5a. The mixture was then concentrated on a rotary evaporator to yield the crude product. The solid thus obtained was triturated with CH2Cl2 to yield 3.22g of pure 5a as an almost colorless solid (96% yield). MS (M-H): 290.3. 1H NMR (DMSO): δ 11.75 (s, IH), 7.88 (dd, IH), 7.32 (m, 4H), 7.21 (dd, IH); The PPl-RlOOO (2000L GL reactor) reactor was charged with 3a (64.4 Kg, 1.0 eq), anhydrous tetrahydrofuran (557 Kg) and triethylamine (2.2 Kg, 0.1 equiv). The charging line of 2000L GL reactor was rinsed with tetrahydrofuran (10 Kg). The contents of the reactor were agitated for 25 mins. during that period complete solution was obtained. The PPl- R2000 (200L HP reactor) reactor was charged with N-Boc-p-phenylenediamine (38 Kg, 1.0 equiv), tetrahydrofuran (89 Kg) and agitated for 30 mins. until complete solution obtained. The contents of the 200L HP reactor were transferred to the 2000L GL reactor containing the compound 3a and then heated at 65 +/- 5 0C for 2 hrs. The reaction was deemed complete monitored by HPLC after confirming the disappearance of starting material 3a (in-process specification < 1 %). The contents of 2000L GL reactor were cooled to 20 +/- 5 0C and then charged with sodium methoxide (25% solution in methanol, 41.5 Kg, 1.05 equiv.) over 20 mins. maintaining the temperature below 30 0C. The charging lines were rinsed with tetrahydrofuran (10 Kg). The contents were agitated at 25 +/- 5 0C for 4 hrs. In-process HPLC analysis confirmed the completion of the reaction when the amount of compound 3b remaining in the reaction mixture is < 1%. To this reaction mixture added filtered process water (500 Kg) and distilled under vacuum the 2000L GL reactor contents into clean 200L GL receiver until 300 Kg of solvent is distilled. The solids obtained were filtered using GL Nutsche filter and washed with process filtered water until the color of the solid the compound 4b is white to grayish. The 2000L GL reactor is charged with wet compound 4b filter cake, dioxane (340 Kg) and agitated the contents for 1 hr. The filterable solid obtained were filtered through GL Nutsche filter with a sheet of T-515 LF Typar filter paper. The solid cake was blow dried for 2 hrs and then charged with dioxane (200 Kg) into the 2000L GL reactor. The contents were agitated for 10 min. and then charged with 4 N HCl in
References:
WO2007/56167,2007,A2 Location in patent:Page/Page column 37; 41-42

207346-42-7
155 suppliers
$11.00/1g

936500-99-1
22 suppliers
inquiry