
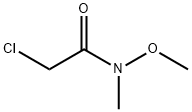
Ethanone, 1-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-chloro- synthesis
- Product Name:Ethanone, 1-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-chloro-
- CAS Number:937047-12-6
- Molecular formula:C8H7ClN4O
- Molecular Weight:210.62
![7-bromopyrrolo[1,2-f][1,2,4]triazin-4-amine](/CAS/GIF/937046-98-5.gif)
937046-98-5
175 suppliers
$11.00/250mg
67442-07-3
197 suppliers
$6.00/5g
![Ethanone, 1-(4-aminopyrrolo[2,1-f][1,2,4]triazin-7-yl)-2-chloro-](/CAS/20210111/GIF/937047-12-6.gif)
937047-12-6
1 suppliers
inquiry
Yield:937047-12-6 84%
Reaction Conditions:
Stage #1: 7-bromo-pyrrolo[2,1-f][1,2,4]triazin-4-ylaminewith chloro-trimethyl-silane in tetrahydrofuran at 20; for 3 h;
Stage #2: with isopropylmagnesium bromide in tetrahydrofuran at 40; for 1.33333 h;
Stage #3: 2-chloro-N-methoxy-N-methylacetamide in tetrahydrofuran at -10 - 20; for 0.5 h;Product distribution / selectivity;
Steps:
J.1
A 1 L, 3-neck RB flask was fitted with a mechanical stirrer, a nitrogen inlet, thermocouple and thermocontroller, and a water cooling bath. In the flask 7-bromopyrrolo[2,1- f][1 ,2,4]triazin-4-amine (175 g, 352mmol) was suspended in tetrahydrofuran (100 ml_) and treated with chlorotrimethylsilane (7.65g, 70.4 mmol). The mixture was allowed to stir 3 h at rt. A solution of isopropylmagnesium bromide in THF (2M, 70.4 mL, 141 mmol) was added slowly over 20 min taking care that the internal temperature never rose above 40 0C. After 1 h a sample quenched in MeOH and analyzed by RP-HPLC indicated that the metallation was > 90% complete. The water bath was replaced with an ice-acetone bath and stirring was continued until the internal temperature fell to -10 0C. 2-Chloro-N- methoxy-N-methylacetamide (7.3 g, 53 mmol) was added as a solid, and the reaction allowed to warm to rt over 30 min. The reaction was quenched with MeOH and diluted with EtOAc (200 mL) and 500 mL of citrate buffer (pH 4). The mixture was stirred for 15 min, during which time a thick tan precipitate formed. This was filtered off to provide the desired compound as a tan solid (2g, 27%). The organic layer was separated, dried with sodium sulfate and filtered through a silica plug. Removal of the solvent in vacuo and trituration of the residue with EtOAc gave a second batch of the desired product (4.3 g, 58%). The 2 batches were combined to provide the desired product as a tan powder (6.25 g, 84%). 1H-NMR (DMSO): δ 7.88 (bs, 1 H), 7.80 (s, 1 H), 7.08 (d, 1 H, J = 5 Hz), 6.70 (d, 1H, J = 5 Hz), 4.82 (s, 2H), 3.06 (bs, 1); MS: LC/MS (+esi): m/z=211.2 [MH]+ ;LC/MS rt = 1.69 min.
References:
WO2007/56170,2007,A2 Location in patent:Page/Page column 84-85